Adhesives function by the following mechanisms:
Mechanical Adhesion

http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/chemical.gif
- The adhesive penetrates a porous substrate, locking itself mechanically to the substrate.
- For an adhesive to mechanically adhere to a substrate, it
must have the right physical properties to infiltrate the
substrate.

http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/interlock.gif
Chemical Bonding
- Chemical bonds form across the interface between an adhesive and a substrate.
- Primary chemical bonding forces have much higher energies
that other, secondary forces.

http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/chemical.gif
Electrostatic Adhesion

http://documents.specialchem.com/documentsas/indexables/contents/4/images/electrostatic.gif
- Differences in electronegativities of adhesive
materials compared to a substrate creates an adhesive
force.
- The adhesive force is created to the transfer of
electrons across the interface, which creates positively
and negatively charged surfaces.

http://documents.specialchem.com/documentsas/indexables/contents/4/images/electrostatic.gif
Adhesion by Absorption

http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/absorption.gif
- Adhesion results from intermolecular contact between two materials due to surface forces that develop between the molecules of the two surfaces.
- The most common surface force consists of van der Waals forces.
- Van der Waals forces are a result of correlations
in the fluctuating polarizations of nearby
particles.

http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/absorption.gif
- Van der Waals forces are directly influenced by the wettability of surfaces and surface tension.
- For a contact angle of θ°, surface wetting is described by Young's Equation:
γLA⋅Cosθ=γSA−γSL.
(N/m)
(Adhesive
surface tension) *
COS(θ) = (Substrate
surface energy) - (Adhesive surface tension)
- For proper adhesion, good wetting must occur.
- Complete wetting occurs when:
γSA>
γLA
Adhesion by Diffusion
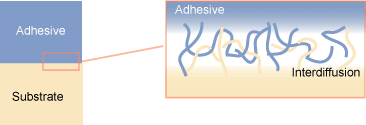
http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/interdiffusion.gif
- Polymeric materials may adhere to each other
due to the interactions between polymer chains
at their interface.
- The entangling of polymer chains is affected by contact time, temperature, chemical properties, and physical form.

http://www.specialchem4adhesives.com/documents/indexables/contents/4/images/interdiffusion.gif
Continue to: FAILURE OF ADHESIVES