Binding Energy
As stated before, low Z atoms are candidates for fusion, whilst high Z atoms are ones in which fission is their nuclear reaction. But why is this the case? If you take a look below, you will see a graph called the Binding Energy Curve. This graph shows the binding energy of the element's nucleus with Fe (Iron) being the strongest and most stable and elements descending from this peak on either side. The important thing to take away from this graph is that anything to the left of iron is going to have fusion as its nuclear reaction, and anything to the right of iron will be capable of fission. This is an important concept for you can see that isotopes of hydrogen like deuterium and tritium are quite low on the binding energy curve and do not have stable bonds between the sub-atomic particles of their nucleus. All elements wish to have their nuclei in a more stable part of the graph i.e. iron being the most stable, and so will attempt to move up the graph towards that goal as a result of their nuclear reaction. Here is the Binding Energy Curve: 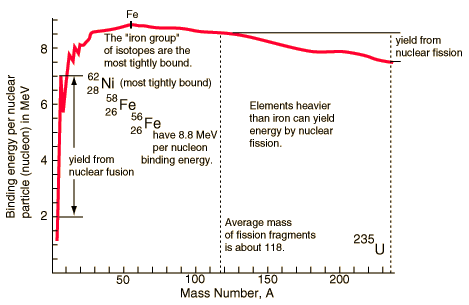
Courtest of: http://hyperphysics.phy-astr.gsu.edu/hbase/nucene/nucbin.html
|
Fusion
Fusion is a fascinating nuclear reaction in which two low atomic number (Z) atoms have their nucleus combined to create a single atom. This type of reaction is very common in the universe as every star in known existence uses fusion to create the light and heat energy emanating from it. The Sun is actually a gigantic fusion power generator giving our relatively small planet both heat and light allowing for the diversity and abundance of life found on our planet. The heat at which fusion reactions take place are many order of magnitude higher than fission, as a fusion reaction requires millions of degrees K! (This is talked about in greater depth later.) However, aside from the pressure and temperature concerns associated with sustained fusion power, as the Sun will tell you, it is a powerful source of energy. Below is a picture of deuterium and tritium fusing to create a Helium-4 isotope and a free neutron:
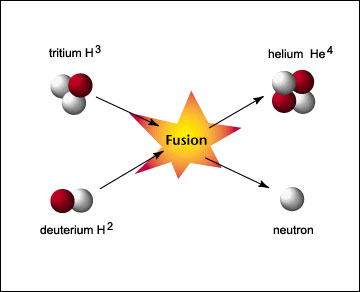 Courtesy of: http://www.atomicarchive.com/Fusion/Images/fusion.jpg |
 Courtesy of: http://www.newcastle-schools.org.uk/nsn/chemistry/Radioactivity/fissionanim.gif
Courtesy of: http://www.newcastle-schools.org.uk/nsn/chemistry/Radioactivity/fissionanim.gif
 Courtesy of: http://www.atomicarchive.com/Fusion/Images/fusion.jpg
Courtesy of: http://www.atomicarchive.com/Fusion/Images/fusion.jpg