Cathode Ray Experiments

1st Experiment
http://home.clara.net/don.ainley/john.htm
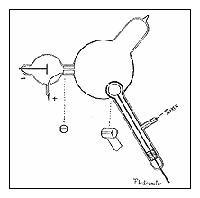
- J.J. Thomson built a cathode ray tube with a pair of metal cylinders with a slit. These were connected to an electrometer to measure electrical charge.
- He experimented to see if the charge can be separated from the rays by bending the rays with magnets.
- Once discovering he could not separate the two, he concluded that the negative charge and the cathode rays are somehow connected.
2nd Experiment
http://home.clara.net/don.ainley/john.htm
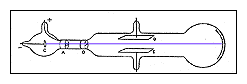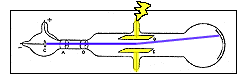
- Charged particles normally curve while moving through an electric field unless surrounded by a conductor. Thomson wondered if the traces of gas left in the tube were being turned into a conductor by the cathode rays.
- He extracted as much of the gas from a tube as he could and learned that the cathode rays did bend in an electric field.
- From these two experiments he concluded that cathode rays are charges of negative electricity carried by particles of matter.
3rd Experiment
http://home.clara.net/don.ainley/john.htm
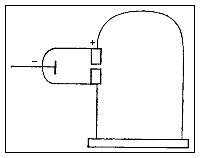
- The objective of this experiment was to learn the basic properties of the particles.
- He measured the extent to which the rays were bent by a magnetic field and how much energy they carried using various tubes and different gasses.
- He was then able to calculate the ratio of the mass of a particle to its electric charge (m/e).
- The mass to charge ratio was calculated to be over one thousand times smaller than the ration of a charged hydrogen atom.
- Thomson concluded from this that the matter in the cathode rays were in a newly found state, one in which all matter is made of.