Electron
tunneling is the non-intuitive process of an electron passing
through a barrier
that is usually deemed to be impassable. To understand this
(well, kind of - see above), you need to remember that matter
exists as both a particle and a wave. Remember? Awesome.
Now picture yourself at the base of a steep
hill. In front of you is a ball. For reasons unknown, you want
to get the ball on the other side of the hill, but lack the
energy to push it over the top. From a classical (Newtonian)
physics point of view, that's it. Party's over. If you don't
have the strength to push the ball over the hill, then the
ball will stay on this side of the hill.
However quantum physics allows for a more
subtle approach: matter exists as both a particle and a wave,
right? You remembered! Now in the previous situation, we
talked about the ball as made up of many small particles.
Carbon atoms forming long polymer chains forming a rubber
ball. But if matter is both a particle and a wave, then this
ball must also exist as a wave function. At this point, you
should probably scroll up and reread the first quote: "Nobody
knows how it can be like that," so don't worry. If this is
true, and it is, then this wave-function ball can interact
with the barrier in a totally different way. Instead of
requiring a certain quota of energy to push it up over the top
of the hill, the ball can travel through the hill. It can tunnel.
At this point, it might be helpful to have
a visual:
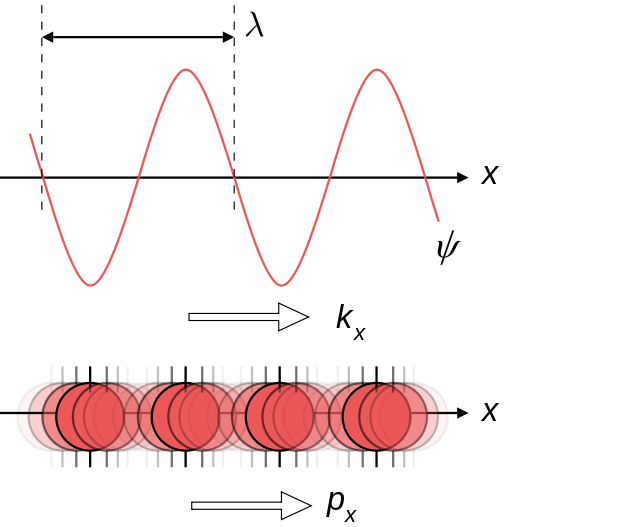 Particles of matter exist as
probabilities described by a wave function. The probability of
finding a certain particle in space is higher at the peaks and
troughs of the wave. The lower axis illustrates this by
darkness of coloration: areas where the ball is dark are high
probabilities, whereas areas where the ball is light are areas
where the particle has a low probability of being found.
Particles of matter exist as
probabilities described by a wave function. The probability of
finding a certain particle in space is higher at the peaks and
troughs of the wave. The lower axis illustrates this by
darkness of coloration: areas where the ball is dark are high
probabilities, whereas areas where the ball is light are areas
where the particle has a low probability of being found.
The
Heisenberg Uncertainty Principle (don't worry), states that
the more precisely you try to pin down a particle's location
or momentum, the more elusive its location or momentum become.
You can know one thing, but you can't know more than one thing
at one time. This can be used for a bit of trickery: locations
of the particle exist as probabilities, therefore the particle
has a non-zero probability of being anywhere. Some locations
may be exceedingly improbable (approaching infinitely
improbable), but there's still a chance it will be there.
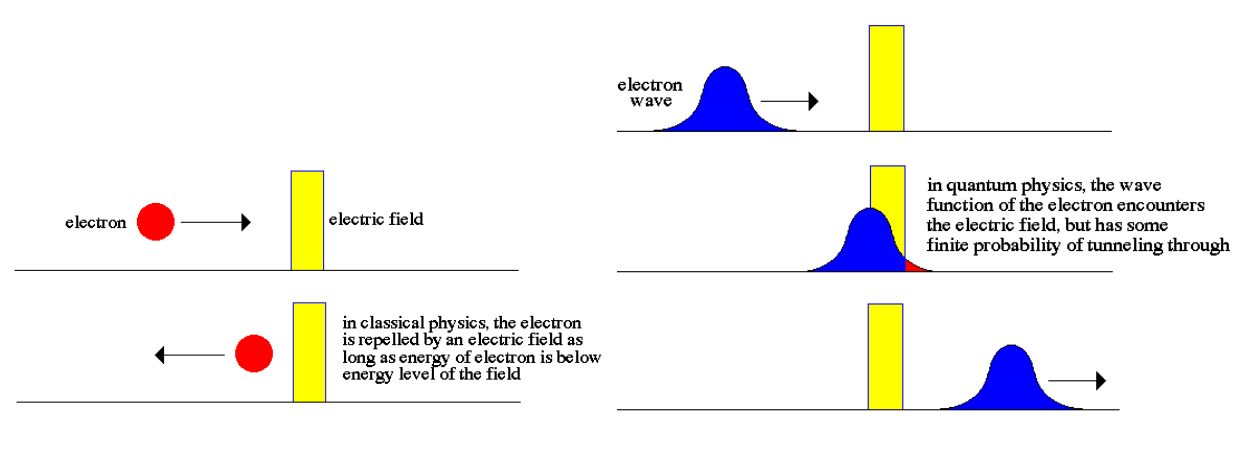
So,
back to our hillside: though the ball may lack the energy to
surmount the hill, there is another way. Though very small,
there exists a
probability that when the ball encounters the hill (remember
the ball's a particle and
a wave) it will not roll up the hill, but will instead 'exist'
on the other side by means of probabilities. It will pass
through the hillside without appearing to have physically done
so. For lack of a better term, this process is called
'tunneling.'
Don't
worry if this doesn't make sense (scroll up and re-read the
quotes), just know that particles such as electrons can pass
through barriers by some tricky methods and that the mechanism
for doing so is a very strange one.
(cc) Maschen
Fig 1. Matter existing as both a
wave (upper axis),
and a probability (lower axis).
Opaqueness of the
particle on the lower axis illustrates
the probability
of existence (dark = more probable).
Classical Physics:
Quantum Physics:
Dr. James Shombert, University of Oregon
 The
Physics of Smell
The
Physics of Smell The
Physics of Smell
The
Physics of Smell
 Particles of matter exist as
probabilities described by a wave function. The probability of
finding a certain particle in space is higher at the peaks and
troughs of the wave. The lower axis illustrates this by
darkness of coloration: areas where the ball is dark are high
probabilities, whereas areas where the ball is light are areas
where the particle has a low probability of being found.
Particles of matter exist as
probabilities described by a wave function. The probability of
finding a certain particle in space is higher at the peaks and
troughs of the wave. The lower axis illustrates this by
darkness of coloration: areas where the ball is dark are high
probabilities, whereas areas where the ball is light are areas
where the particle has a low probability of being found.