
The Carnot Cycle of Refrigeration |
According to Knight (chapter 21), the definition of the carnot cycle and a carnot engine is "any Carnot engine operating between TH and TC must have exactly the same efficiency as any other carnot engine operating between the same two energy reservoirs."
Tests have been done to understand the refrigeration and cycle, as given by Coker.
Detailed in the cycle, the system starts with an isothermal compression from the compressor, while energy is released. The next step, from the compressor to the condenser, the refrigerant is expanded adiabatically to the "cooler" temperature. Afterwards, energy from the deposit (cold) area is transferred to the refrigerant and is compressed adiabatically back to the disposal (hot) reservoir.

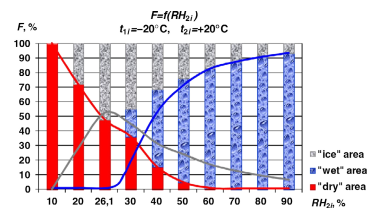 (figure 5, Patil et al, pg.1077)
|
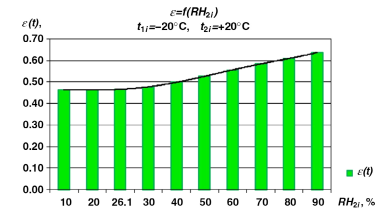 (figure 4, Patil et al, pg. 1076) |
| The Processes | References |