The
Modern Atom- What's
different?
Studies of the atom
have surely come a long way from the times of
Democritus, Dalton, and Mendeleev. Thanks to a
compilation of brilliant minds, we have a much
greater understanding of the modern atom. Through
further discoveries, quantum mechanics has become
greatly more complicated and diverse. The behavior
of particles is now understood, protons and
neutrons are no longer elementary particles, and
multiple atom models exist simply to make different
aspects of quantum mechanics more understandable.
Further Electron Understanding:
 While the idea that
electrons are negative and are found outside of
the atom remains, quantum mechanics has further
defined their behavior and where they are located
in space. Albert Einstein discovered that
electrons and photons can act like both waves and
particles. Physicists also discovered that
electrons are located and in areas called electron
shells around the nucleus. These shells have
shapes as shown to the right and the direction of
an electron's spin can even be found. While the idea that
electrons are negative and are found outside of
the atom remains, quantum mechanics has further
defined their behavior and where they are located
in space. Albert Einstein discovered that
electrons and photons can act like both waves and
particles. Physicists also discovered that
electrons are located and in areas called electron
shells around the nucleus. These shells have
shapes as shown to the right and the direction of
an electron's spin can even be found.
Elementary Particles:
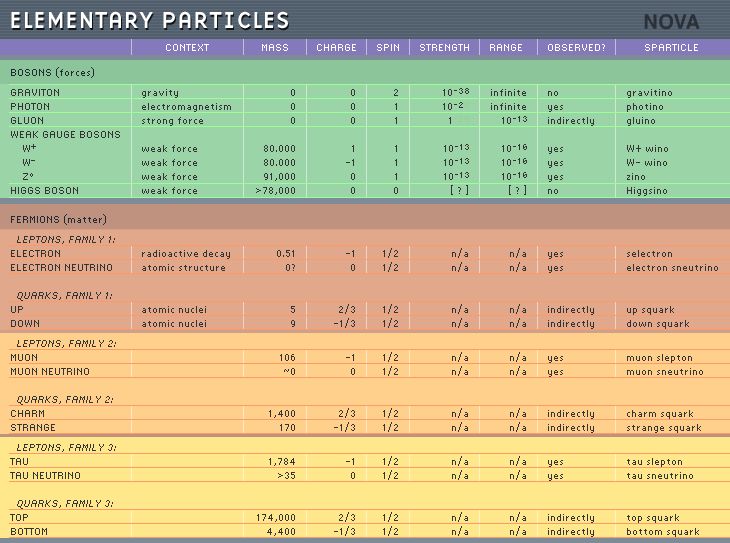
The original
elementary particles were electrons, neutrons,
and protons. Through further research,
dozens of other particles have been
discovered. Bosons, gluons, neutrinos,
gravitrons, and quarks are just a few
names of the many new elementary
particles. Much has yet to be discovered
about these particles but evidence of them
has been proven through particle detectors
and particle colliders. Most of what is known
about these particles is that many of them
help make up greater particles like
neutrons and protons and the elementary
particles are also responsible for
different properties the particles may
have. For example, gravitrons are believed
to be responsible for the gravitational
force between subatomic particles.
|