
| Bullet
Thermodynamics The thermodynamics of a gun are unique for a couple reasons. One is that it is a combustion process that is known to have too little oxygen for complete combustion. This is because within the chamber of the gun, there is not enough volume to hold the propellant and the correct amount of air. For this reason, oxidizer has to be included in the propellant. Along with the oxidizer, there must be fuel in the gunpowder. The fuel comes from charcoal and sulfur. The charcoal provides carbon to the reaction, and the sulfur provides fuel, as well as lowers the ignition temperature of the gun powder. Charcoal (C) has an ignition temperature of 350oC, whereas sulfur (S) has an ignition temperature of 200oC. The oxidizer used in gun powder is potassium nitrate (KNO3), which allows the combustion to happen in the absence of air. A basic balanced equation is as follows: 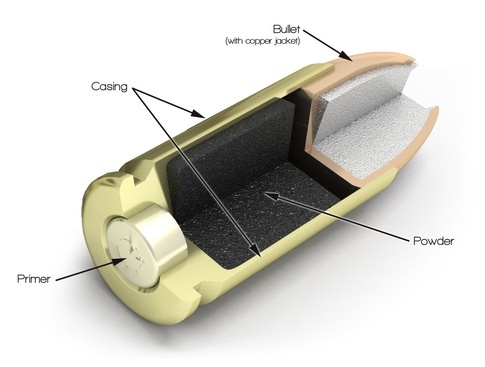 Picture Source
In order to set off the gun powder, it must be
ignited. This is the function of the primer. The primer contains a small amount of lead styphnate, which is an extremely sensitive explosive. It is so sensitive that a change in pressure can set it off. When the firing pin hits the primer, the lead styphnate gets pressed between the anvil and the new indentation made by the firing pin. This sends flame through the cartridge and ignites the gunpowder.   Picture Source
Gunpowder has about 3MJ of energy per kg,
which comes out to 3000 joules per gram. One gram is equal to roughly 15.43 grains, which is the unit gunpowder is measured in. A standard .45 ACP round has between 4.5 and 6 grains of powder. This means a standard .45 ACP load has between 875 and 1166 joules of energy inside of the casing. Looking back at the first section for the kinetic energy of the bullet, ~477 joules, we can, roughly, determine the efficiency of the system. This gives the system an efficiency between 41% and 55%. |
Title Page Introduction Recoil Reloading Bibliography |