Ring Current

- Since the pi electrons in these
p-orbitals are delocalized, they can be thought
of as being shared by every carbon of the
aromatic ring
- Since the electrons can move
from on carbon p-orbital to the overlapping
carbon p-orbital without changing the compound
the electrons are free to move in a circle in
the overlapping p-orbitals around the aromatic
ring.
- This circular movement around
the aromatic ring by the electrons causes a
current to be formed by the moving electrons
because the negative charged electrons are
moving from one carbon to the next.
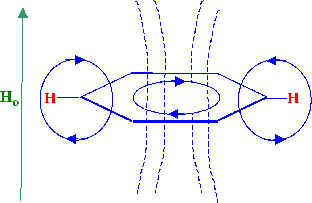
http://www.analyticalspectroscopy.net/ap7_html_7d5bd661.gif
The inside circle of the benzene ring shows the
direction of current caused by the pi electrons going
around the ring.
The current resulting from a benzene ring is I=(-ne^2H0)/4pi
m c
where n is the number of electrons, e is the charge on
the electron, H0 is the
strength of a perpendicular magnetic field
|
