Resonance structures

- Aromatic ring structures, like
benzene, have p-orbitals that overlap that are
filled on one side and empty on the other
- The pi electrons that are in
these p-orbitals can be shifted as seen through
resonance structures
- Resonance structures are the
same compound but with the electrons moved from
one carbon to the other
- Since the pi electrons can
freely move from one p-orbital to another, seen
through the moving of bonds in a resonance
structure, the electrons are considered to be
delocalized
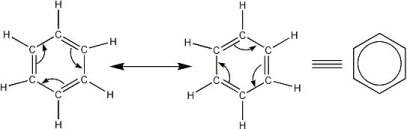
http://www.armstrongwynne.org/benzene.html
This image shows how the pi electrons of benzene can
be moved around to form resonance structures.
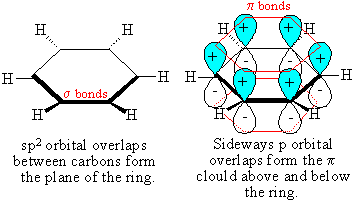
http://chemistry.gowengray.com/Tutorials/orbitals/c6h6keku.gif
Shows the p-orbitals filled on one side but not on
the other and how they overlap.
|