The Fission Process

Fission involves the process of a large nuclei splitting into
smaller nuclei, either through a nuclear reaction of radioactive
decay. Binding energy is related to how stable certain nuclei are,
with the largest being seemingly the most unstable, the following
graph shows which nucleons are best suited for fission or fusion,
with the heaviest nucleons being the best for fission.
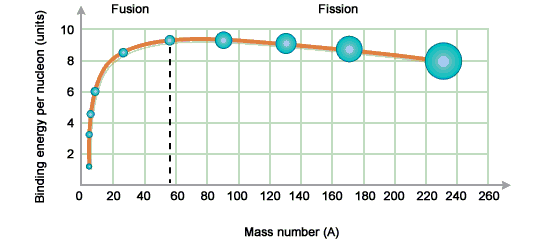
(http://www.bbc.co.uk/bitesize/higher/physics/radiation/nuclear_reactions/revision/2/)
The heavier nucleons are inherently unstable, and as a result
naturally want to split into two smaller, more stable atoms through
the process of fission. However, this process takes millions of
years in nature, so something must be done to induce these reactions
artificially. This leads to the use of neutron bombardment to induce
the same reaction that would have occurred naturally. When a large
nuclei absorbs an additional neutron it may become unstable and
split into two smaller nuclei and additional free neutrons. An
example of a reaction like this would be Uranium-235 absorbing a
neutron and then splitting into one Barium-139 atom, one Krypton-94
atom and 3 free neutrons. Barium and Krypton are only one of the
hundreds or possible products from the neutron induced Uranium-235
reaction. The following graph shows the distribution of fission
fragments based on atomic mass, where the green area represents the
fission of uranium-233, blue of plutonium-239, red of uranium-235,
and black a mixture of uranium and plutonium.
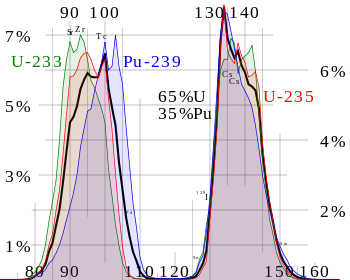
(http://en.wikipedia.org/wiki/Nuclear_fission_product#/media/File:ThermalFissionYield.svg)
Barium and Krypton are only one of the hundreds or possible products
from the neutron induced Uranium-235 reaction. These reactions also
release an enormous amount of energy in the form of kinetic energy
from the fission products, gamma ray emmision, and fast-neutrons.
“The induced fission of this isotope releases an average of 200 MeV
per atom, or 80 million kilojoules per gram of Uranium-235. The
attraction of nuclear fissionas a source of power can be understood
by comparing this value with the 50kJ/g released when natural gas is
burned”[2]. Nuclear reactors use the
additional free neutrons that are released to induce additional
reactions in what is known as a nuclear chain reaction. These chain
reactions are controlled within a nuclear reactor, which then
harness the energy to produce power. In contrast, in nuclear weapons
the chain reaction is left uncontrolled.

Next: The Fusion Process
Previous: Introduction
Title Page

