Buoyancy in The Atmosphere.
Buoyancy is a phenomenon that occurs when substances of varying density meet. Displacement is a mechanism of Buoyancy, and is ultimately due to gravity. Take for example, a bucket of dirt. This bucket of dirt has stones from the size of tennis balls down to grains of sand the size of salt. If you shake the bucket enough, the large stones find their way to the bottom and the sand finds its' way to the top. Following the same mechanism, buoyancy naturally places less dense objects on top of more dense objects.
An object's density is defined by how much a certain volume of it displaces.
If you are a baker, Volume can be thought of as a "gallon" or a "cup." Weight can be thought of as a "pound" or an "ounce". Water has a higher density than flour. However, those units of measurement are not so nice to deal with outside the realm of cupcakes. For our purposes, we will be using the SI unit system. (eg, Meters (M) and Kilograms (Kg))
Density is defined as:
where ρ is the density, m
is the mass, and V is the volume.
Water has a density of 1000
Kg/M^3, and air has a density that varies with
temperature and pressure, but is roughly 1kg/m^3.
That said, If you have a sealed and rigid box of
volume 1m^3 and you place it in water, that box will be
able to support roughly 1000Kg before it sinks.
In dealing with air and other gasses,
their properties are simplified by using a standard
temperature and pressure. (STP). At room temperature
and pressure (0 C and 100 kpa) The density of air is 1.27
kg/m^3.


Now for the fun part. If you heat air, the little
molecules that are bouncing around and making it have the
density that it has start to gain speed. Therefore, they
can be in more places in less time and it essentially
takes less hot air molecules to take up the same
volume than colder ones.
The Ideal Gas law governs volume,
pressure, amount of molecules, and temperature.
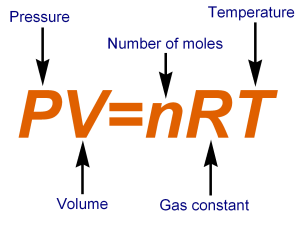
n, the number of moles can essentially be re-written into mass by multiplying by the molar mass of air. (28.97 g/mol.).
Now that we have volume on one side of the equation and mass on the other, we can re-write the ideal gas law to solve for density.
Density =(Pressure(atm)(28.97))/(8.31)(Temperature (kelvin).)
A sample calculation at 0c and 100kpa gives:
[(100)(28.97)]/[(8.31)(273.15)] = 1.27 (kg/m^3)
The most common fabric of a hot air balloon is 1.3oz/square meter silicone-impregnated rip-stop nylon, and so a hot air balloon has a maximum envelope temperature of about 120c before the nylon stats to degrade. figuring on a safe operating temperature of 100c, a hot air balloon has a density of .93kg/m^3. this leads to a lift of .34kg/m^3. in comparison to hydrogen at stp, with a density of .09kg/m^3, with a lift of 1.18kg/m^3, hot air produces about 30% the lift of hydrogen. However, it is a heck of a lot easier to heat air than it is to produce a large amount of hydrogen.
A MAXIMUM CEILING?
As elevation is gained, the density and temperature of the surrounding air steadily decreases. Therefore, the lift of a hot air balloon steadily decreases, until the density of the hot air inside the balloon is equal to the density of the surrounding air, and neutral buoyancy is reached. What height does this happen at?
The height record set for a hot air balloon was set by Vijaypat Singhania in 2005, and sits at 69,000 feet. ( a little over 21,000 meters!)