|
How
Matches Work

Lets start
with a look at Safety Matches. These are the
ones that come in a container with a
designated striking surface. The head of a Safety Match
contains Sulfer, glass powder, and an
oxidizing agent such as Potassium Chlorate.[7]
The
striking surface is made of glass powder,
sand, and Red Phosphorus. The Potassium Chlorate is
used to create oxygen for the flame, the
Sulfer used as a fuel, and the glass and
sand used to create a rough surface for
better friction.
"Safety Matches." Photo. Coghlans.com.
Coghlan's Ltd. n.d. Web. 15 Apr. 2014.
|
When the match head
is struck across the striking surface, the
friction creates enough heat to revert the
Red Phosphorus back to White Phosphorus,
which then immediately ignites from contact
with the air. The resulting fire of this
reaction uses the Potassium Chlorate to
expand into a larger flame which then
ignites the Sulfer. Then, as the Sulfer
burns up the wood of the stick catches fire.
This all happens in a short burst of fiery
life when you light a match. The video below
shows the process described in at a slower
rate so that you can better see the
reaction.
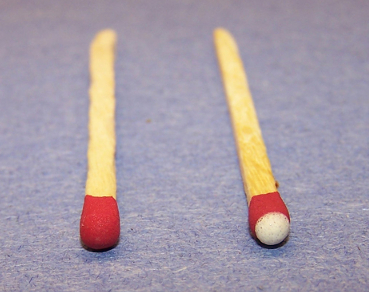
So what's the
difference between a Safetey Match and a
Strike-Anywhere match? Its as simple as
putting the Phosphorus in the match head
instead of on the container. For these
matches, Phosphorus Sesquisulfide is used
because it is easier to ignite with friction
than Red Phoshorus. You can see the
difference between the two, as shown in the
image below. Strike-Anywhere Matches have a
tip of Phosphorus, whereas the Safety Match
is one color.
Boundreaux, Kevin. "Safety
Match and Strike-Anywhere Match."
Photo. Angelo.edu. n.d. Web.
15 Apr. 2014.
|
|