Energy Potential

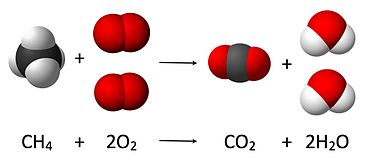 Combustion Reaction
Combustion Reaction
- Because methane hydrate is
composed of methane gas, the solid is
flammable. According to the Britannica
Encyclopedia, a combustion reaction is one
that burns organic carbon molecules with the
presence of oxygen. The result is a more
stable carbon compound (carbon dioxide),
water, and heat.
- The Conservation of Energy states that
energy can not be created or destroyed, but
only transferred from once source to
another. In the same way, the large amounts
of heat (energy) does not come out of
nowhere
- Instead, the methane molecule has the
potential to create heat
- Differs from Potential Energy that
is reliant on height and gravity
- The process is due to the valence
electrons that are transferred in the ion
shell of the carbon atoms
- According to the book, Physics For
Scientists and Engineers: A Strategic
Approach, the author, Randall D.
Knight, states that, " Energy can be
transferred between the system and the
environment if they have thermal
interaction. The energy transferred in a
thermal interaction is called heat."
|