This website will focus on molecular magnetism as it takes place in a crystal lattice structure. Magnetism at the molecular level arises from interactions between unpaired electrons in the compound. Each electron has a magnetic moment determined by the electron spin, angular momentum, and the strength of an applied magnetic field. Most of the time these spins are randomly oriented and the substance will behave as a para-magnet. However, at low temperatures the magnetic moments of each election can align in a specific orientation. If they align parallel the interaction is ferromagnetic, if they align anti-parallel, the interaction is anti-ferromagnetic. The temperature at which this occurs is called the ordering temperature[1].
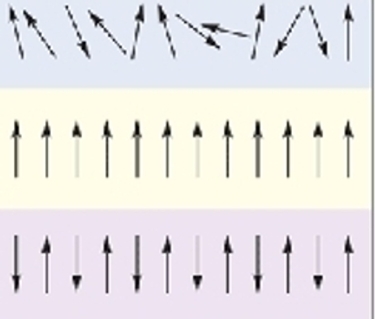
Figure 1: Examples of spin orientations, starting from the top, paramagnetism, ferromagnetic, and anti-ferromagnetic.
 |
There are many factors affecting the magnetic properties of these types of systems. Some of them are described here:
- Dimension- Magnetic interactions occur in multiple directions. For example in a three dimensional lattice you can have a 2-D interaction that exists in each layer of the lattice. However, if there is magnetic interaction between the layers, the interaction is described as three dimensional.
- Frustration- Frustration occurs when there are multiple spin configurations of the same energy possible. This causes the spin to oscillate between the available states because all are equally acceptable.
Figure 2: Diagram of possible equal energy spin states for a planar three spin system.
 |
- Anisotropy- Anisotropy describes the difference in magnetization depending on how the crystal is oriented with respect to the applied field. This must be taken into account when determining the magnetic dimension of the lattice.


