{image borrowed and modified from: http://antibubble.com/ }
Have you ever watched water fall on water and noted with amazement that sometimes a tiny droplet
will dance across the surface of the water before joining it? When such a droplet becomes entirely
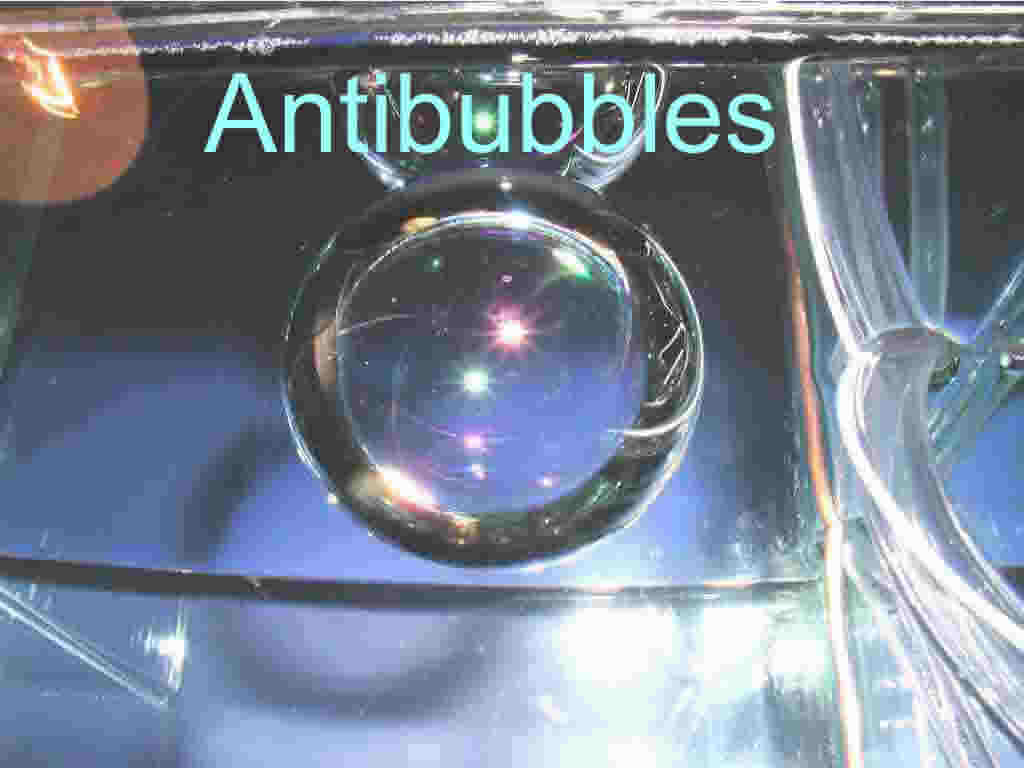
submerged while retaining a pocket of air about it an antibubble has formed. These curious creations
are essentially the opposite of a soap bubble in air, that is they are a film of gas immersed within a
liquid. These antibubbles are much more difficult to create than are ordinary bubbles, though they
result when a portion of a liquid (call it liquid A) falls into another body of liquid (liquid B) and a
"skin" of air is retained about liquid A as it enter the other liquid. In practice this is usually
accomplished by adding a surfactant to the entering liquid so that it tends to hold itself togethor
(check out the figure below){9}.
{ Image borrowed and modified from: http://www.antibubble.org/jchang/index.html }
The properties of these peculiar objects are very complex and apparently only relatively recently
have scientists begun to study them (they were discovered in 1932 {XI}), thus the physics of these
antibubbles is not currently satisfactorily understood{X.}. Researchers are currently attempting to
describe how they form and collapse, they've even gone so far as to create these antibubbles in
Belgian beer for the "fun" of it {XI, XII, XIII}. For more information regarding these bubbles visit
the works cited for this page.