Heat Pumps and Refrigerators

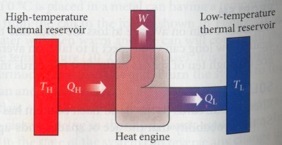
A heat pump is a heat engine that absorbs energy from a thermal source TH, converts the energy in work, and rejects the remaining heat to a lower temperature thermal sink TL. A heat engine follow the first law of thermodynamics such that QH = QL + W. The efficiency of a heat pump is defined as:
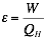
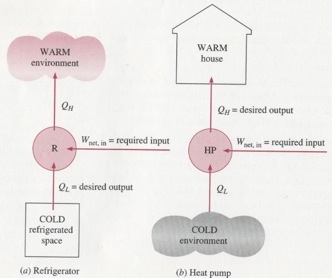
A heat pump is a heat engine typically used for air conditioning. A heat pump pulls energy from the lower thermal reservoir and rejects it to the higher reservoir. Because this is counter to a natural flow between two temperature gradients, additional work from an independent source is required to run the cycle. The desired output for a heat pump is the heat rejection (typically to a building). Work can be derived from a heat pump, but that the desired quantity. A “coefficient of performance” can be calculated as
K=QL/W
the added heat per required work.
A refrigerator works in the same fashion of a heat pump, except the desired quantity is the heat absorption from the colder reservoir. The coefficient of performance for a refrigerator is:
K=QH/W