
Page 2:
Schools of Thermodynamics
The study of thermodynamics is separated into two branches: the classical and the statistical thermodynamics.
Classical thermodynamics was the original study of thermodynamics in early 1800s. It was concerned with thermodynamic states, and properties as energy, work, and heat, and with the two laws of thermodynamics. However, classical thermodynamics lacked an atomic interpretation of the processes. Classical thermodynamics derives from the research done by physicist Robert Boyle. He developed the concept that the pressure P of a given quantity of gas varies inversely to its volume V at constant temperature. In other words this equation was derived: PV = k, a constant. From here, the thermo-science began to develop with the construction of the first successful atmospheric steam engines.
The first and second laws of thermodynamics emerged simultaneously in the 1850s.
With the development of atomic and molecular theories in the late 19th century, thermodynamics was given a molecular interpretation, which the classical thermodynamics lacked. This field is called statistical thermodynamics, which can be thought of as a bridge between macroscopic and microscopic properties of systems. Statistical thermodynamics is focused around the macroscopic results. The statistical approach is to derive all macroscopic properties (temperature, volume, pressure, energy, entropy, etc.) from the properties of moving particles and the interactions between them in the given system. Statistical thermodynamics was found to be very accurate and successful; therefore it is widely used by scientists around the world.
Page 3:
History
Thermodynamics is a branch of physics which deals with the energy and work of a system. It was born in the 19th century as scientists were first discovering how to build and operate steam engines. The term thermodynamics was first used by James Joule to express the relationship between heat and power.
The history of thermodynamics begins with a German scientist who designed and built the first vacuum pump. Shortly after that development, Robert Boyle built an air pump. Using this pump, Boyle noticed the pressure-temperature-volume proportionality. With this new development Boyle's Law was formulated, which states that pressure and volume are inversely proportional.
The little developments in pumps and piston engines lead to the invention of an engine. The first engine was invented by Thomas Savery. Although these early engines were crude and inefficient, they attracted the attention of the leading scientists of the time. One such scientist was Sadi Carnot, the "father of thermodynamics” (Carnot Engine). He marks the beginning of thermodynamics as a branch of science. He created the Carnot Cycle and designed an ideal engine called the Carnot engine. This engine has the highest achievable efficiency an engine can have. Sadi Carnot, in 1824, published “Reflections on the Motive Power of Fire”, a research paper on heat, power, and engine efficiency. The paper outlined the basic relations between the Carnot engine, the Carnot cycle, and locomotive power (“Thermodynamics”). The Heat Engine and the Carnot inventions are discussed further on.

This is a typical engine or thermodynamic system. Heat moves from hot boiler to cold condenser and work is extracted.
http://en.wikipedia.org/wiki/Thermodynamics
Page 4:
Systems and Processes
Isolated Systems – matter and energy may not cross the boundary.
Adiabatic Systems – heat may not cross the boundary.
Diathermic Systems - heat may cross boundary.
Closed Systems – matter may not cross the boundary.
Open Systems – heat, work, and matter may cross the boundary.
(book reference)
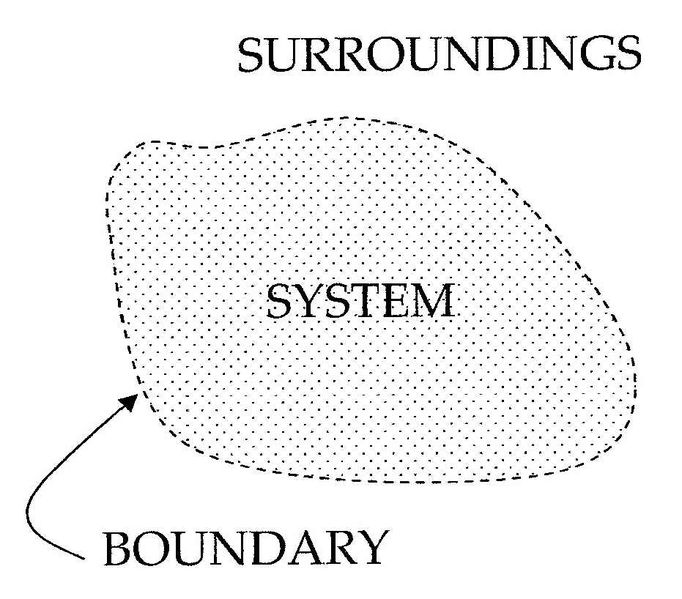
http://en.wikipedia.org/wiki/Image:System-boundary.jpg
Page 5:
Systems and Processes (continued)
An isothermal process occurs at a constant temperature.
An isobaric process occurs at constant pressure.
An isometric or isovolumetric process occurs at constant volume.
An adiabatic process occurs without loss or gain of heat.
An isentropic process occurs at constant entropy.
An isenthalpic process occurs at a constant enthalpy.
(book reference)
The first three processes are the most common process used to describe systems.
A more general diagram of a thermodynamic process is shown below.
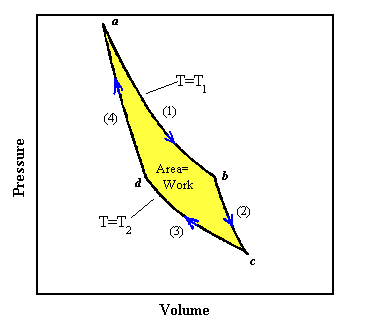 http://electron9.phys.utk.edu/phys136d/modules/m3/images/carnot.gif
http://electron9.phys.utk.edu/phys136d/modules/m3/images/carnot.gif
Page 6:
Heat flows from a high temperature TH furnace through the working body (working substance) and into the cold reservoir TC. This process forces the working substance to do work W on the surroundings
http://en.wikipedia.org/wiki/Carnot_engine

http://en.wikipedia.org/wiki/Carnot_cycle
ΔW is the work done by the system (energy exiting the system as work),
ΔQH is the heat put into the system (heat energy entering the system),
TC is the absolute temperature of the cold reservoir, and
TH is the temperature of the hot reservoir.
The above equation gives the maximum efficiency possible for any engine using the corresponding temperatures. The efficiency can never equal one however. It is not possible as defined by the laws of thermodynamics.
Page 8:
Carnot Cycle (continued)

The above diagram represents a Carnot cycle acting as a heat engine. The process is illustrated on a temperature-entropy diagram. The cycle takes place between a hot reservoir at temperature TH and a cold reservoir at temperature TC.
http://en.wikipedia.org/wiki/Carnot_cycle
Isothermal expansion of the gas at the hot temperature, TH. Steps A to B on the diagram - the expanding gases cause the piston to do work on the surroundings. The gas expansion is propelled by absorption of heat from the high temperature reservoir.
Adiabatic expansion of the gas. Steps B to C on diagram - no heat is gained or lost. The gas continues to expand, doing work on the surroundings. The gas expansion causes it to cool to the cold temperature, TC.
Isothermal compression of the gas at the cold temperature, TC. Steps C to D on the diagram. Now the surroundings do work on the gas, causing heat to flow out of the gas to the low temperature reservoir.
Adiabatic compression of the gas. Steps D to A on the diagram. The surroundings do work on the gas, compressing it and causing the temperature to rise to TH. At this point the gas is in the same state as at the start of step 1.
(book reference)
Page 9: Laws of Thermodynamics
There are 3 laws of thermodynamics: the zeroth law, the first and second laws of thermodynamics.
The ZEROTH LAW states the following:
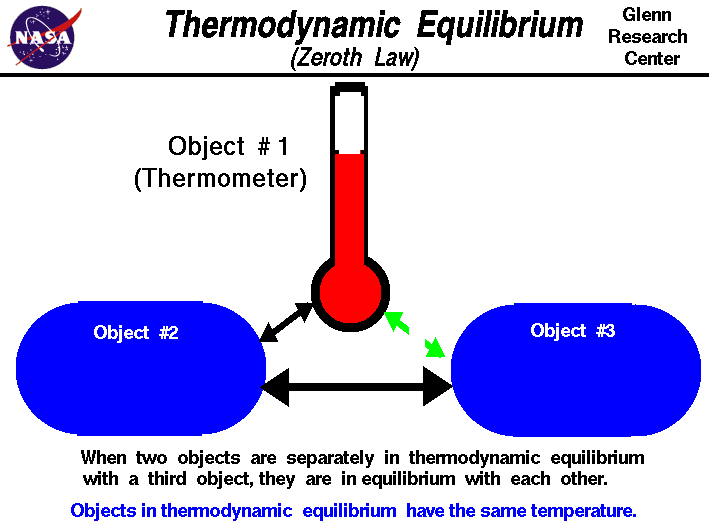
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo0.html
The FIRST LAW of thermodynamics is about conservation of energy. The energy can never be created or destroyed: ΔE = Q + W. The change in the internal energy of a closed thermodynamic system is equal to the sum of the amount of heat energy supplied to the system and the work done on the system.
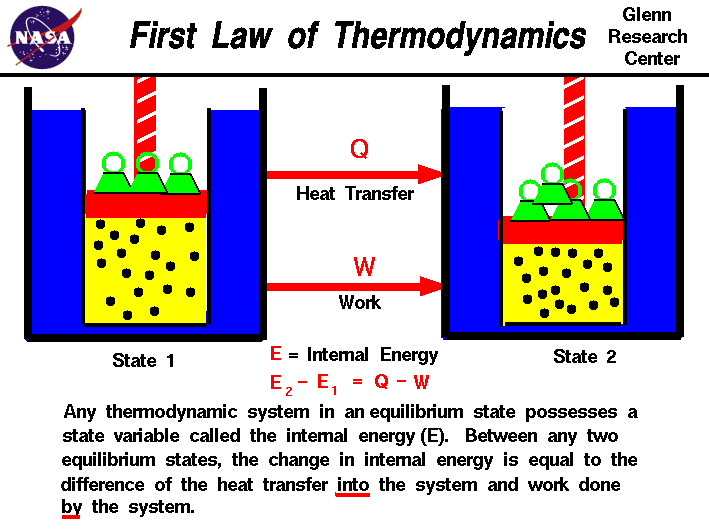
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo1.html
The SECOND LAW of thermodynamics is about entropy. The total entropy of any isolated thermodynamic system tends to increase over time, approaching a maximum value. It also states that the maximum efficiency of an engine is never equal to 1 and the maximum possible efficiency is approached by a Carnot engine.
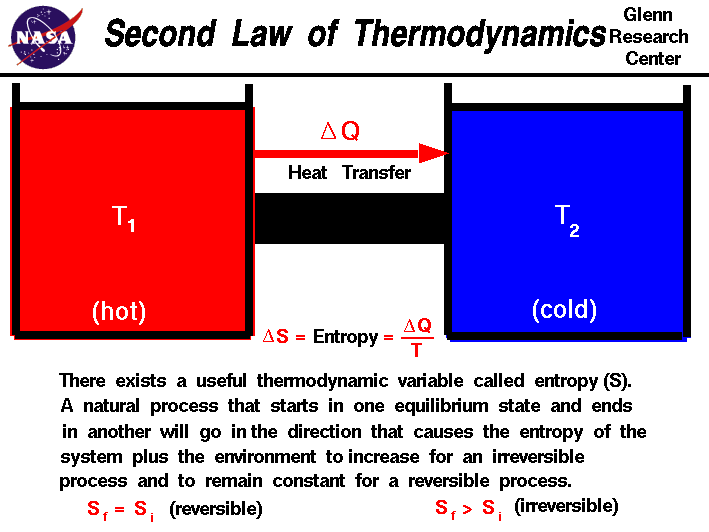
http://www.grc.nasa.gov/WWW/K-12/airplane/thermo2.html