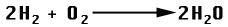The Basics of Hydrogen

- Hydrogen by itself is not dangerous, but it becomes explosive whenever oxygen is present! 1
Taken from Dangerous Labratories.
- Many of us are familiar with Hydrogen as it is used to propel spacecraft. 2
Taken from Google Images.
- Comparing the reaction for burning Hydrogen as opposed to burning methane gives: 3
Hydrogen + Oxygen = Water
Image taken from Utah State Office of Education
http://www.usoe.k12.ut.us/CURR/Science/sciber00/8th/matter/sciber/chemtype.htm
Energy Released in the
combustion of 1.15g H2 =
(11.3 kJ/ºC)(14.3ºC) = 162 kJ
Simplifying this value for 1g of H2 = 162 kJ / 1.5g = 141 kJ/g
Simplifying this value for 1g of H2 = 162 kJ / 1.5g = 141 kJ/g
verses:
Methane + Oxygen = Carbon Dioxide + Water
CH4 + 2O2 → CO2 + 2H2O
Image taken from Wikipedia
http://en.wikipedia.org/wiki/Methane
Energy Released in the
combustion of 1.5g CH4 =
(11.3 kJ/ºC)(7.3ºC) = 83 kJ
Simplifying this value for 1g of CH4 = 83 kJ / 1.5g = 55 kJ/g
Simplifying this value for 1g of CH4 = 83 kJ / 1.5g = 55 kJ/g