Methods of Heat Transfer
![]()
There are 3 basic types of heat transfer that will be discussed herein: Conduction, Convection, and Radiation.
![]()
Conduction is the transfer of heat through a given material via excitation of individual atoms or molecules, which in turn transmit some of their vibrational energy to adjacent atoms or molecules, which in turn, continue the process. Various materials conduct with a variety of rates, with some materials being relatively good conductors, (for instance: metals, glass, and ) while others are relatively poor conductors (e.g. Asbestos, fiberglass, cork, and expanded or extruded polyurethane.)
In general, gasses are rather poor conductors because the distance between each molecule is relatively large. (This does not always hold true for compressed gasses.) Metals and Metallic alloys are relatively good conductors because they normally possess a large number of free electrons which are at liberty to travel through the material. These free electrons support the thermal conductivity of the material as they can carry energy over relatively large distances both easily and expediently.
Conduction only occurs when there is a temperature difference between two sections of a material that are in thermal contact. (Note that thermal contact may be thought of as being in actual physical contact AND/OR being in close enough proximity that the heat energy of the objects are not isolated from one another.)
In conduction, there are essentially four components to calculating the power (generally in units of Watts or BTU/hr) transferred through a given area.
| Symbol | Component | Description | |
| The Area Itself | This area is the area through which the heat flux is being calculated. | ||
| The Temperature Difference | The Temperature difference is the difference in temperature, from one side of the material or medium in question to the other. | ||
| The Coefficient of Thermal Conductivity | The proportionality constant empirically determined and found in published tables. --- Table for Common Materials | ||
| The Material Thickness | This thickness is the breadth of the material perpendicular to the plane of the area. |
These components are utilized in Fourier's Law of heat conduction to calculate the rate of heat conduction.
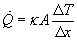
Also useful is the rate of heat conduction per unit area calculated as follows:
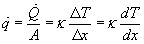
These tools are useful in calculating heat loss through a given region, e.g. a wall, window pane, floor, etc.
![]()
Convection is the transfer of heat energy between a solid surface and an adjacent liquid or gas that is in motion. In reality it is based on a combination of fluid motion and conduction. As the velocity of the fluid motion increases, the faster the rate of cooling. Without the presence of bulk motion in the fluid medium, heat transfer is governed purely by conduction.
There are 2 types of convection, forced and natural. In forced convection, a fan or other device (can include wind) is utilized to rapidly cool an object, whereas natural convection is entirely dependent upon the fluid motion induced by buoyancy forces created by variations in the density of the fluid medium.
In convection, there are essentially 3 components to calculating the power (generally in units of Watts or BTU/hr) transferred through a given area.
| Symbol | Component | Description | |
| The Surface Area | This surface area is the region through which the heat transfer takes place. | ||
| The Temperature Difference | The Temperature difference is the difference in temperature of the bulk fluid and the surface which is losing heat. | ||
| Convection Heat Transfer Coefficient | This value is an empirically determined parameter. Common value ranges for h can be found in the Convection Table |
These components are utilized in Newton's Law of cooling to calculate the rate of heat loss due to convection.
![]()
Also useful is the rate of heat convection per unit area calculated as follows:
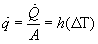
![]()
Radiation is the energy emitted by matter in the form of electromagnetic waves, and unlike conduction and convection, radiation does not require the presence of a medium between the surfaces engaged in the heat transfer.
Radiation is a volumetric phenomenon, though for opaque surfaces (e.g. Metals, wood, and rocks) it is considered to be a surface phenomenon as the radiation emitted by the interior regions of the material can never reach the surface.
In radiation, there are essentially 6 components to calculating the power (once again, generally in units of Watts or BTU/hr) transferred between two surfaces.
| Symbol | Component | Description | |
| The Surface Area | This surface area is the surface area of the emitter. | ||
| The Temperature of Surface 1 | The Temperature difference is no longer a key issue, so much as the temperature of each surface, this temperature is simply that of one of the radiant surfaces (absolute). | ||
| The Temperature of Surface 2 | The Temperature of the second radiant surface (absolute scale). | ||
| The View Factor | The View Factor is the value that relates the amount of the surfaces that are in thermal contact with each other. (For instance, someone feels warmth on one side from a campfire, but cold on the side not facing the flames). | ||
| The Emissivity | Emissivity is simply a value between 0 and 1, that is a material property that indicates the willingness of a material to radiate energy. | ||
| The Stefan-Boltzman constant | 5.67E-8 |
These components are utilized in the Stefan Boltzman Law to calculate the rate of heat loss due to radiation.
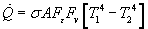
Also useful is the rate of heat loss due to radiation per unit area calculated as follows:
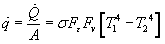
Common Thermal Conductivities k |
Common Thermal Resistance-{R} Values
|
Emissivities |
| Aluminum | 238 | 1' Hardwood Siding | 0.91 | Aluinum Foil | 0.07 | ||
| Iron | 79.5 | 4" Brick | 4.0 | Anodyzed Aluminum | 0.82 | ||
| Asbestos | 0.08 | 3.5" Fiberglass Insulation | 10.9 | Polished Copper | 0.03 | ||
| Concrete | 0.8 | 6" Fiberglass Insulation | 18.8 | Black Paint | 0.98 | ||
| Glass | 0.8 | 0.25" Flat Glass | 0.89 | White Paint | 0.90 | ||
| Ice | 2 | Cellulose Fiber 1" | 3.7 | Asphalt | 0.85-0.93 | ||
| Water | 0.6 | Lapped Wood Shingles | 0.87 | Human Skin | 0.95 | ||
| Wood | 0.08 | Fiberglass Board 1" | 4.35 | Wood | 0.82-0.92 | ||
| Air | 0.0234 | 3.5" Air space | 1.01 | Soil | 0.93-0.96 | ||
| Hydrogen | 0.172 | 0.5" Gypsum Board | 0.45 | Water | 0.96 |
R and k values from 9. Serway Jewett
Emissivities from 10. Thermodynamics