Scuba diving is all about making sure you get enough air in you while underwater. Simple enough? Its actually complicated because of how air acts under different pressures, depths and in your body and equipment. In order to obtain your scuba certification it is important to know and understand the physics behind the operation; mainly a few associated Gas Laws.
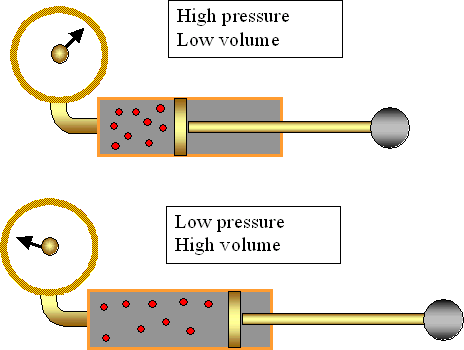
Boyles Law relates the pressure and volume of a gas that is held at constant temperature.
P1V1=P2V2
Where P=pressure, V=volume and the
subscripts 1 and 2 represent the initial and final states.
Essentially what Boyles law is saying is that pressure and volume are
inversely related.
If you increase the pressure then
the volume will decrease; and if you increase the volume then the
pressure will decrease.
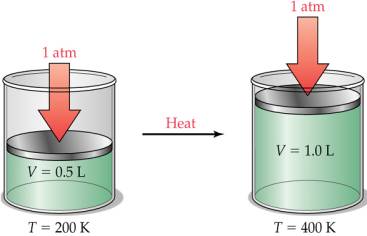
Charles Law
relates temperature and volume at a constant pressure.
T1/V1=T2/V2
Where T=temperature, V=volume
and the subscripts 1 and 2 represent the initial and final
states. Charles Law states that a decrease in temperature will
decrease its volume which also implies that if the volume were to
remain constant there would have to be an increase in pressure.
Imagine it is a beautifully sunny day and you are at the beach, your
scuba gear is packed in your trunk, including your tank and you are
just basking in the sun for a bit (clearly not located in Fairbanks
Alaska). However when you return to your car, your scuba tank has
exploded! Charles law explains why that happened. Your tank
stayed at a constant volume but had an increase in temperature due to
the hot sun, therefore the pressure increased and provided perfect
conditions for an explosion.
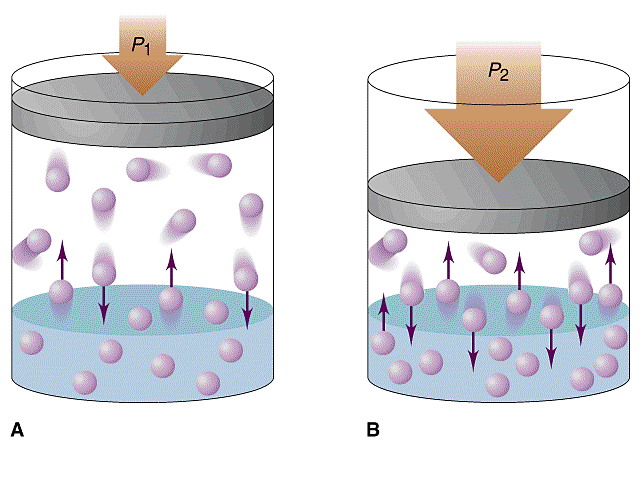
Henry's
Law:
P=KC
Where P=pressure, C is the
concentration of the gas and K is Henry's law constant. This law
tells the diver that at higher pressures our bodies will absorb more
gasses. The deeper the dive, the greater the amount of nitrogen
absorbed into the body. Therfore the greater the depth the
greater the risk of decompression illness.
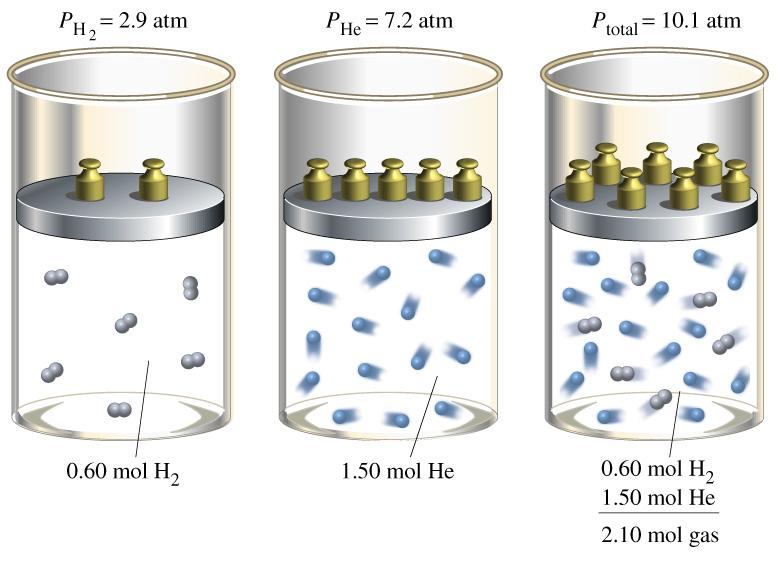
Dalton's
Law
of Partial Pressure simply states that the pressure exerted
by a mixture of gases is equal to the sum of the pressures that would
be exerted by the gases individually.
P=p1+p2+p3+...pN
Where P is total pressure due to the
partial pressures p. This tells us that all gases tend to compress
equally by volume.