Carnot Cycle
The Carnot cycle is a theoretical heat engine cycle that represents the most efficient cycle capable
of converting thermal energy into mechanical energy. The theoretical Carnot cycle ideals may also be applied
to the reverse, turning mechanical work into a heat transfer device, most commonly used in refrigeration. A
lot of useful information can be obtained by comparing the Carnot cycle to real life heat engine cycles.
Temperature vs. entropy diagrams and temperature vs. pressure diagrams (like the one below) are often a good
visual aid in comparing these cycles. Aside from all the technical jargon, one of the most amazing facts
derived from Carnot cycle analyses is that a heat engine can never be more efficient than the Carnot efficiency
of an engine operating on the same temperature gradient. Below we will discuss these ideas in more depth.
hhttp://upload.wikimedia.org/wikipedia/commons/thumb/0/06/Carnot_cycle_p-V_diagram.svg/400px-Carnot_cycle_p-V_diagram.svg.png
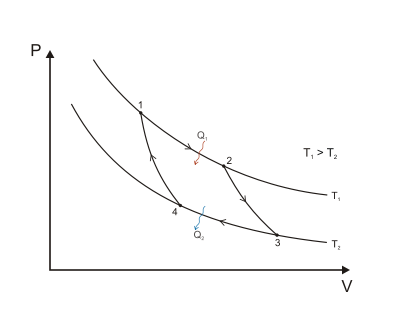
The above diagram is a Pressure vs. Temperature diagram of a Carnot cycle, the ideal heat engine. Let us analyse the cycle:
From state 1 to state 2: This process is isothermal(no change in temperature), while heat is added to the system. Notice that this is the high temperature.
From state 2 to state 3: This process is adiabatic(no heat exchanged in or out of the system), and the temperature drops to the low temperature.
From state 3 to state 4: This process is also isothermal but heat is removed from the system and it is isothermal along the low temperature.
From state 4 to state 1: This process is also adiabatic, temperature increases and allows us to arrive at our initial state where the cycle can start over again.
The amount of work this system can theoretically output(or the amount of work required to complete this cycle in reverse)
is the area inside this cycle. As you can see analysing this diagram can be very useful.
This leads us to our next and most important aspect of the Carnot cycle, efficiency.
Efficiency of a Carnot cycle is described by the equation:
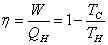
Where n is the efficiency, W is work, Q is heat, and T is temperature in Kelvin.
This truely is amazing because this puts a theoretical limit on how efficient any heat engine can be.
To give this some perspective, lets say your car engine gets up to 400F(about 200C or 473K) and it is
-100F(about -70C or 200K) outside, the maximum theoretical efficency for these conditions is only 58%
efficient! Keep in mind these are very extreme temperatures and would not realistically occur in real
world scenarios. So next time someone says that they have built a 98% efficient heat engine you will
know that they are not telling the truth.
Home
...............
Next
