Anything but a Bohr...
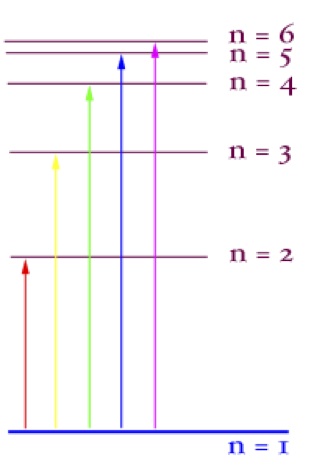
You will also recall that the energy carried by electrons is characterized
by discrete levels, or quanta. When a photon strikes an electron in the photosystems
of a plant’s chloroplast, an electron is “excited” and rises in energy. This energy
can be given off again, in the form of light, or as energy to other systems (such as in
the passing of energy in photosystems.)
http://www.brooklyn.cuny.edu/bc/ahp/LAD/C3/C3_elecEnergy.html
Niels Bohr was the first scientist to describe these levels of energy, the principal
quantum number, also denoted “n”, in the following equation:
En = - Eo/n^2
This is essentially a statement about the amount of energy carried by an electron
dependent upon its quantum location.
http://www.brooklyn.cuny.edu/bc/ahp/LAD/C3/C3_elecEnergy.html