Coulomb is perhaps most famous for the law of physics bearing his name. Coulomb's law (Equation 1) describes the relationship between force, charge and distance. In 1785, Coulomb published a paper describing the torsion balance. This paper would become the first of a series of seven papers that Coulomb would have published on the topics of magnetism and electricity. The torsion balance allowed Coulomb to make more precise measurements of force than anyone prior to that time. Several other researchers, including Henry Cavendish, Joseph Priestly, and Charles Stanhope were similar work related to electrostatics. In fact, independently Henry Cavendish had determined experimentally the electric force was an inverse square law around the same time. However Cavendish never published his results or experiments, so it was not until 1879 that this fact was discovered by James Maxwell. While Priestly's work with electrical repulsion served as the basis for Coulomb's research.
Equation 1-Coulomb's Law
Coulomb's law says the electrical force between two charges (q1)
and (q2) is proportional to the product of the two charges
divided by the distance between the two charges squared (see Equation 1
above). The torsion balance which Coulomb invented, allowed him to
accurately measure electric forces and thereby establish this
relationship. Since the charges q can be either positive or negative, Coulomb's
law implies that the resultant force can be either attractive or
repulsive. Figure 1 illustrates this fact.
Figure 1- Opposites Attract / Similar Charges Repel
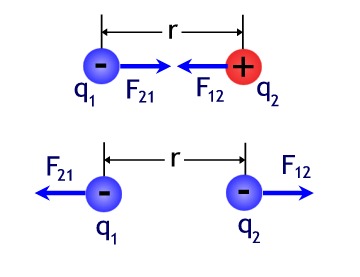
Image by Don Bahls based on Serway Figure 23.6
Torsion Balance
The torsion balance works by isolating
the electric charge (using insulators) and converts the resultant force
into torque allowing it to be easily and accurately measured. Coulomb's
torsion balance worked by charging two pith balls, one of which was fixed
and one of which was
Image 1- Schematic of the Torsion Balance
|
attached to end of needle.
This needle was
attached to a silver wire, with the wire being attached to a torsion
micrometer (See Image 1). This configuration allows the electrical force to be
determined by transferring the force into torque. Whereas
Coulomb's predecessors developed tools which merely indicated the presence
of charge, the torque balance allowed Coulomb to make quantitative
measurements of charge. |
| Image from: http://fargo.itp.tsoa.nyu.edu/~lewis/electricity/pages/coulumb.html | |
Image 2- Torsion Balance

Image from: http://www.fis.uc.pt/museu/129ingig.htm
|
|
|
|
|
|
|||||
|
|