

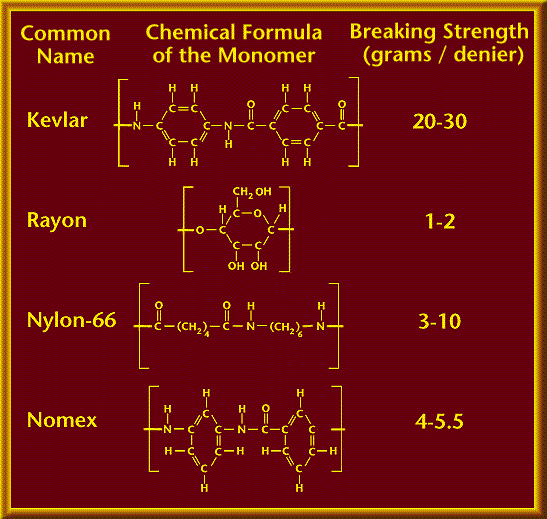
Kevlar is a synthetic (person-made) material known as a polymer. A polymer is a chain made of many similar molecular groups bonded together called monomers.
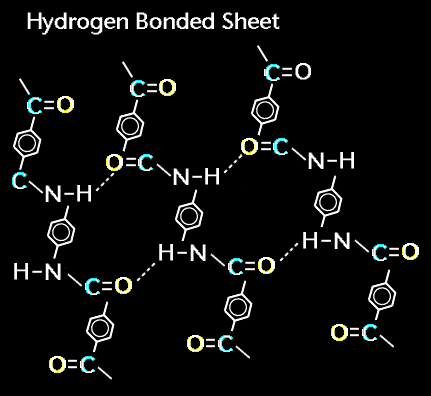
A single Kevlar polymer chain could have anywhere from five to a million segments bonded together. Each Kevlar segment or monomer is a chemical unit that contains 14 carbon atoms, 2 nitrogen atoms, 2 oxygen atoms and 10 hydrogen atoms. The hydrogen bonds greatly strenghten the polymer chain.
 Kevlar is a magnificent material due to the way that it can disperse the energy
and force of an object by its chemical composition. As we know from physics,
the pressure that an object exerts on another object is equal to the force
divided by the area. Kevlar is remarkable at
absorbing and displacing the pressure of an object.
Kevlar is a magnificent material due to the way that it can disperse the energy
and force of an object by its chemical composition. As we know from physics,
the pressure that an object exerts on another object is equal to the force
divided by the area. Kevlar is remarkable at
absorbing and displacing the pressure of an object.