What do
simple ice crystals look like?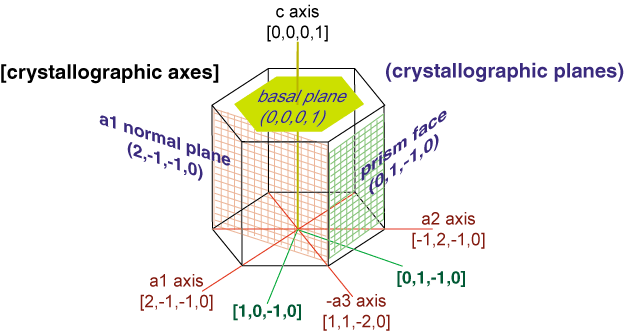
The most basic form of an ice crystal is a hexagonal prism, such as that shown
at right. This form occurs because certain surfaces of the crystal, the growth
facets, grow very slowly. The reason these facets exist derives from the molecular
structure of water, and how water molecules arrange themselves into a crystalline
lattice. (This particular lattice structure is called ice Ih, and is but one
of ice's many different molecular arrangements. However the others occur only
at very low temperatures and/or very high pressures.)
The hexagonal prism includes two hexagonal "basal" faces and six rectangular
"prism" faces. Note that the hexagonal prism can be "plate-like"
or "column-like", if the length along the c-axis is short or long
compared to the length along the a-axes.
What kinds of snow crystals fall from the sky?
Before answering this, it is useful to define what a snow crystal is. Types
of frozen precipitation include:
Snow crystals -- Individual, single ice crystals, often with six-fold symmetrical
shapes. These grow directly from condensing water vapor in the air, usually
around a nucleus of dust or some other foreign material. Typical sizes range
from microscopic to at most a few millimeters in diameter.
Snowflakes -- Collections of snow crystals, loosely bound together into a puff-ball.
These can grow to large sizes, up to about 10 cm across in some cases, when
the snow is especially wet and sticky.
Rime -- Supercooled tiny water droplets (typically in a fog), that quickly freeze
onto whatever they hit. For example, one often sees small droplets of rime on
large snow crystals.
Graupel -- Loose collections of frozen water droplets, sometimes called "soft
hail."
Hail -- Large, solid chunks of ice.
A simple observation on a snowy day, with a low-power microscope or hand magnifying
lens, quickly reveals a great variety of snow crystal shapes. Some different
types include basic plate-like forms



1) Simple sectored plate; 2) Dendritic sectored plate; 3) Fern-like
stellar dendrite
and basic column-like forms:


1) Hollow column, or sheath-like crystal; 2) Needle crystal
More crystal types can be listed, as are described under Classification
schemes. These other forms are mostly variations and combinations of the above
basic types, such as plates with dendritic extensions, capped columns, etc.
Under what conditions do the different
types of snow crystals form?
By growing snow crystals in the laboratory under controlled conditions, one
finds that snow crystals grow in different forms depending mainly on the temperature
and supersaturation level during growth. This is shown in a "morphology
diagram," which gives the crystal shape under different conditions.

From this diagram we see that at very low supersaturation levels, say less
than a few percent relative to ice, crystals grow mostly as simple hexagonal
prisms. The aspect ratio (ratio of sizes along the a-axis and c-axis) varies
somewhat with temperature at low supersaturation, changing from plates (-2 C)
to columns (-5 C) to plates (-15 C) and back to columns again (-30 C). At higher
supersaturation levels the aspect ratios move to more extreme values, and are
strongly temperature dependent. Thus we see long needle-like crystals (a/c <<1)
at -5 C, and very flat plate-like crystals (a/c >>1) at -15 C. At still
higher supersaturations the crystals become more structured; for example at
-15 C one sees sectored plates at intermediate supersaturation levels, and fern-like
dendrites at high supersaturations.
In the sky the supersaturation level is usually at or below the saturation level
of water (the marked line in the morphology diagram); in a cloud the supersaturation
level is fixed near that of water, since the cloud contains a great many small
supercooled water droplets. From the morphology diagram we might thus expect
never to see stellar dendrites in natural snowfall, in contrast to reality.
The solution to this paradox is that snow crystals in the atmosphere are blowing
around, and their motion raises the effective supersaturation level, to the
point where dendrites can form.
Why do snow crystals grow differently at
different temperatures?
The reason we see the above morphology diagram stems from the way ice crystals
grow. For example, at -15 C the basal surfaces of a snow crystal grow very slowly,
while the prism surfaces grow very rapidly, producing plates. At -5 C the prism
surfaces grow more slowly than the basal surfaces, producing columns. The physics
behind this depends on the (temperature dependent) surface structure of ice,
and how vapor molecules are incorporated onto the growing surfaces. This physics
is complex and not well understood, and is the subject of considerable current
research in our own lab and elsewhere.
GO BACK






