Characteristics
of Light
There
are a few fundamental characteristics of light that are
useful to be aware of before proceeding with the discussion
on how CCD's and Film can function to save a useful, meaningful
image. One of these important fundamental qualities is
the fact that visible light is electromagnetic radiation.
Electromagnetic Radiation, Photons, and Energy Levels
Electromagnetic
radiation has many different classifications. Some such
classifications include AM/FM Radio Waves, microwaves,
visible light, x-rays, and gamma rays. A key factor in
these classifications is that each different type or "level"
of electromagnetic radiation contains different energy
levels. These energy levels are determined by the speed
or rate that charges from a given source move to create
an electric field (for instance, moving charges through
an antenna or lightbulb) (Serway 1090). Hence, this oscillating
electric field has two very important characteristics:
it has a frequency and a wavelength. Furthermore, light
can also behave as a particle in some instances. This
particle of light is called a photon, and is essentially
the amount of energy that a light wave has at a
certain frequency (the energy of a photon is not
dependent on the intensity of the light, but rather only
dependent upon its frequency) (Serway 1107). It is this
"duality of light" that allows CCD's and film
to function as they do, as energy is transferred
to materials through light via. photons.
Since
the energy of a photon is only related to its frequency,
an equation (discovered by Einstein) relates photons to
the electrons they produce by:
E
= h * f
Where
E is the energy of the produced electron, h is Planck's
constant (6.63 * 10^-34 J*s), and f is the frequency of
the given light source (CS39J Session Seven 1).
To
understand the wavelengths associated with the different
energy levels of electromagnetic radiation, a plot of
the different frequencies can be seen below in figure
one.
Figure
One (Graphical representation of the Electromagnetic Spectrum)
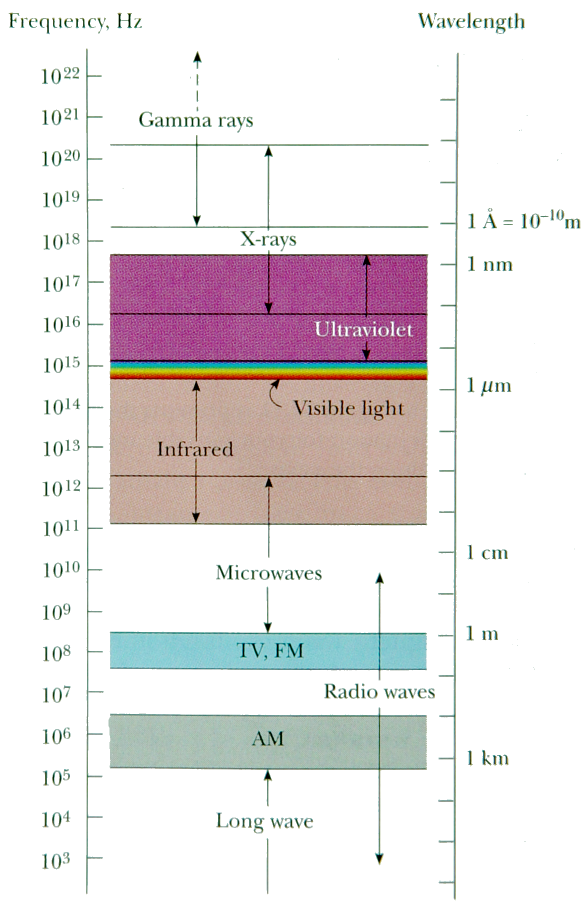
(Courtesy of "Physics for Scientists
and Engineers", Serway, 1094)
In
the above figure, the electromagnetic waves with the lowest
frequencies (and therefore longer wavelengths) are associated
with having lower energies. Also seen from the figure
is the fact that the visible spectrum, the one that most
film, CCD's, and the human eye is calibrated to, has a
wavelength range of 700nm (red) to 400nm (violet) (Serway
1093). A more detailed figure showing the visible spectrum
can be seen below in figure two.
Figure
two ("Magnified" view of the visible spectrum
of light)
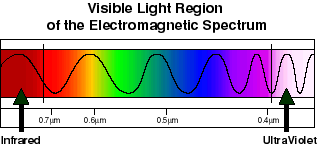
(Courtesy of Visible
Light Waves - The Electromagnetic Spectrum)
From
figure two, it is apparent from the previous discussion
about figure one that the lower energy part of the visible
spectrum is towards the left end (red at a wavelength
of 700nm) and the higher energy region is towards the
right end (violet at a wavelength of 400nm). A prime example
of energy intensities due to higher frequencies can be
introduced here. For instance, the higher energy characteristics
of ultraviolet radiation (shorter wavelength), as opposed
to the lower energy characteristics of infrared (longer
wavelength) is the primary reason as to why ultraviolet
radiation is so damaging to your skin and eyes.
Further
analysis of figure two yields the primary key to
how film and CCD's work. Rather than being sensitive to
all the different wavelengths of light, it only needs
to be generally sensitive to three - red, green, and blue.
From these three colors (and through varying intensities
of each), all shades of color can be obtained from simply
overlapping them. A figure of this type of red/green/blue
color intensity merging can be seen below in figure three.
Figure
Three (Animation of merging colors together with different
intensities of red, green, and blue)
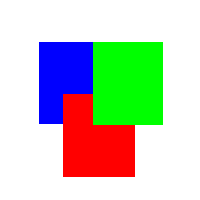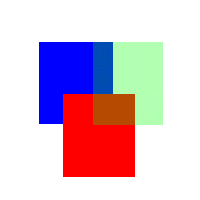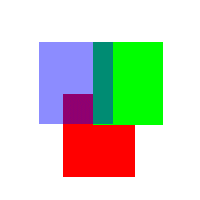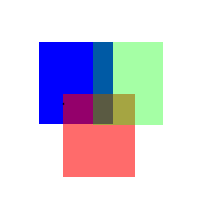
From
figure three, notice the different colors that are appearing
in the overlapping regions of red, green, and blue. In
the case of a faded red on blue or blue on red, the color
is purple. When it's blue on green or green on blue, the
color is more turquoise or dark olive green, and when
it's red on green or green on red, the color shifts towards
more of a brown/orange.
Now
that light has been briefly covered, exactly how a picture
is "setup" can be further explained in the next
section - The Focal Plane.

