|
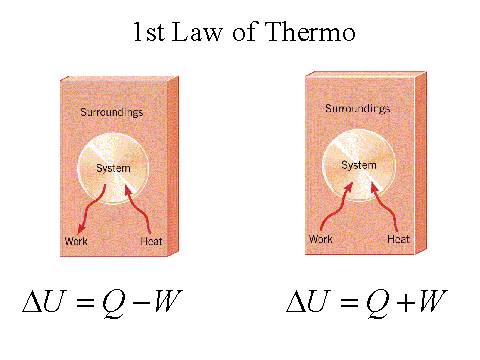
First Law of Thermodynamics
|
|
|
|
www.physics.sfsu.edu/~lwilliam/ 111/thermo/sld006.htm
Stated simply: the total energy of the universe does not change. This does
not mean that the form of the energy cannot change. Indeed, chemical
energies of a molecule can be converted to thermal, electrical or
mechanical energies. DE= q - w Eqn. 1
When q is negative heat
has flowed from the system and when q is positive heat has been absorbed
by the system. Conversely when w is negative work has been done on the
system by the surrounding and when positive, work has been done by the
system on the surroundings. DE = q Eqn. 2 When the same reaction is performed at constant pressure the reaction vessel will do work on the surroundings. In this case: DE = q - w Eqn. 3 where w = PDV Eqn. 4 When the initial and final temperatures are essentially equal (e.g. in the case of biological systems): DV = Dn[RT/P] Eqn. 5 therefore, w = DnRT Eqn. 6 by rearrangement of equation 3 and incorporation of the statements in equations 4-6, one can calculate the amount of heat released under constant pressure: q = DE + w = DE + PDV = DE + DnRT Eqn. 7 In equation 7 Dn is the change in moles of gas per mole of substance oxidized (or reacted), R is the gas constant and T is absolute temperature.
|