SUPERCONDUCTORS
The Two Types of Superconductors
Type 1
Type I superconductors consist
of only one element, either a metal or metalloid. These superconductors
typically have the lowest critical temperatures for which they reach their
superconducting state. Elemental carbon has the highest critical temperature
at 15 K. Note that this is true only when carbon is configured in
a single wall nano-tube. Diamond and graphite does not exhibit superconductivity
. Type I superconductors usually exhibit slight conductivity at room
temperature but have a rather sharp switch to the superconductivity state
when brought to their critical temperature. It is surprising to note
that three of the best conductors known at room temperature, copper, silver,
and gold, have not been found to have any superconducting properties. This
is because a superconductor must have a strong acoustic coupling between
the electrons and the lattice. Good normal conductors by contrast are good
conductors at normal temperatures because they have a weak interaction between
the electrons and the lattice. This weak interaction accounts mainly for
their lack of superconductivity.
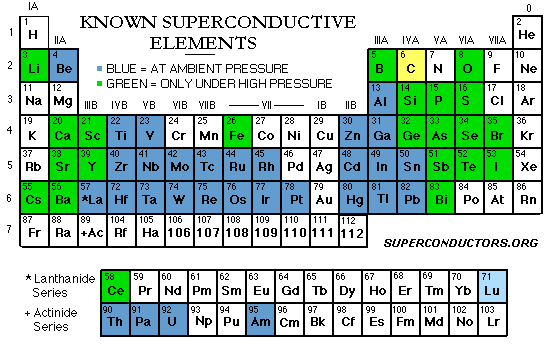
To reach the state of superconductivity, a metal or
alloy must have an average of 2-8 valence electrons. Transition metals average
3, 4·7, or 6·4 valence electrons per atom seem particularly favorable. 2,
4 or 5·6 is unfavorable. It is also known that superconducting elements have
relatively small atomic values.
Type II
Type II superconductors are,
for the most part, comprised of metallic compounds and alloys. This
class of superconductors generally has a much higher critical temperature
than those in Type I. They achieve a higher critical temperature than Type
1 superconductors by a mechanism that is still not completely understood.
It is believed that it relates to the planar layering within the crystalline
structure. The highest critical temperature reached is currently 138
K. Debates still arise as to whether or not an upper limit exists for a
critical temperature to be found.

