 Figure 1
Figure 1 Figure 1
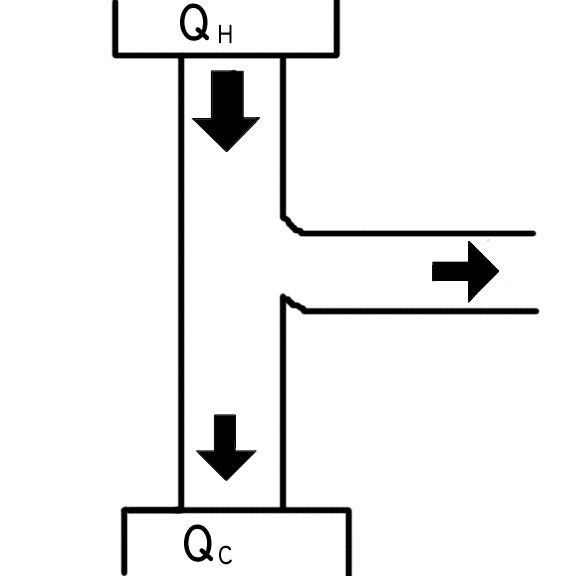
Figure 1 Our illustration in Figure 1 above shows the process
of a typical engine. There is an input of heat, Qh, and there are
outputs of work and heat, W and Qc respectively. This is how the
stirling engines work. There is heat put into the hot side of the
chamber, work done, and heat extracted from the cold side. An ideal
engine functions precisely like this. The only difference in an ideal
engine is that Qc is zero.
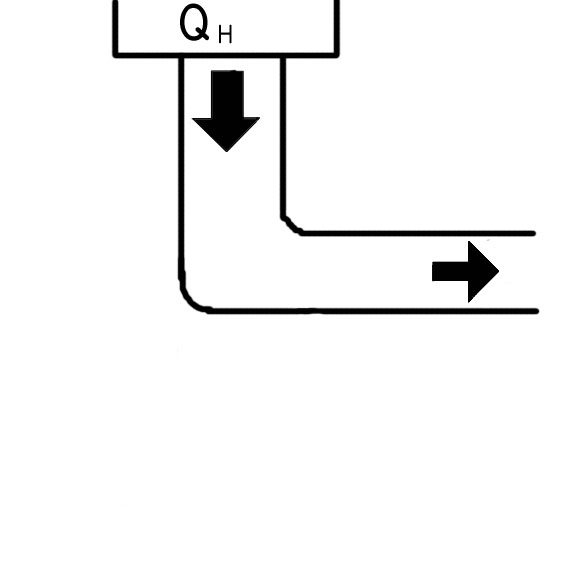 Figure 2
Figure 2
In an ideal engine (Figure 2), all of the energy put into the system as heat, is transformed into work, with no loss. This would mean (as seen on our common equations page) that the efficiency of an ideal engine is 100%.