Thermodynamics
Conduction
| The
heating element underneath the lava lamp plays a
huge role in how it behaves. Since the wax's density
is so close to that of waters, merely heating it up
will change the density enough for it to become
lighter than water. In order for the wax to get hot
in the first place thermal conduction must happen.
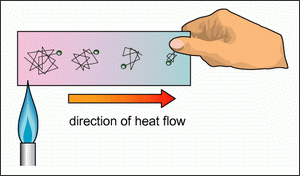
Thermal conduction happens when two bodies are in
contact with each other, the hotter of these two
will transfer heat to reach thermal equilibrium.
This is because on a molecular level particles run
into each other like billiard balls, the hotter body
will have more collisions than the colder body, when
these two bodies are put in contact some of that
kinetic energy between molecules is transferred to
the slower molecules, thus heat is transferred to
the colder body. With lava lamps, the heating
element has to heat the glass (an excellent
conductor of heat) then the glass heats the wax.
Once this wax is heated enough it will get less
dense due to thermal expansion. When a body is
heated it has the ability to change volume, this is
because the particles within a substance begin
moving around a higher velocities and thus maintain
a greater average separation than normal. As the wax
thermally expands it becomes lighter than water and
begins to rise, thus beginning the thermodynamic
processes of convection. |
 Example of conduction-http://www.hk-phy.org/contextual/heat/hea/condu/conduction_e.gif |
 An example of convection-http://www.uoguelph.ca/geology/geol2250/glossary/HTML%20files/convection.jpg |
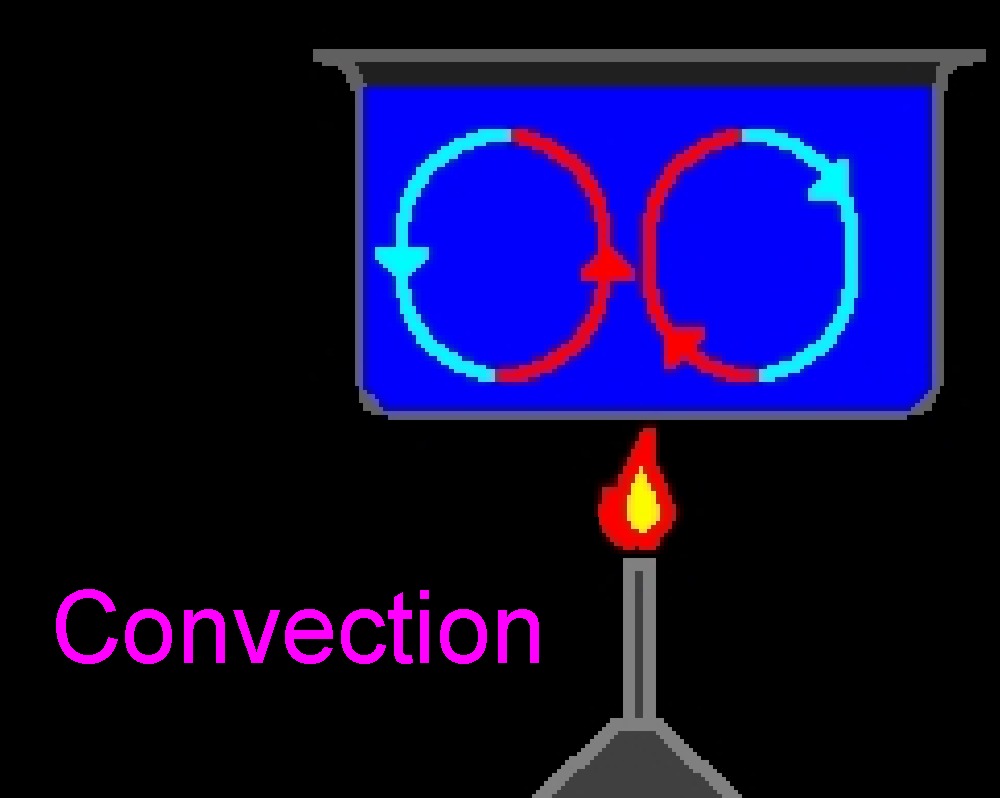
Convection,
the transfer of heat through a liquid by
circulation of currents, is very apparent in lava
lamps. When the wax is heated it becomes less
dense than its surroundings and rises. As the wax
travels up through the water it begins to cool, by
dissipating heat into its surroundings, and by the
time it reaches top it begins to sink again. This
is also happening with the water but since its
clear we cannot see it. |