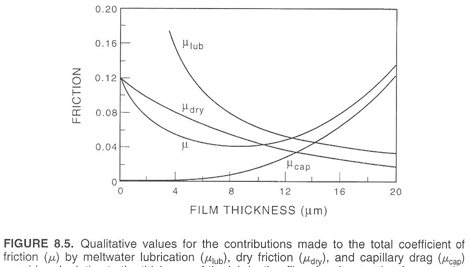
Strangely enough, a ski’s friction is what allows it glide. The friction from the ski gliding over melts some snow creating a thin layer of water, which acts as a lubricant for the skier. The energy needed to melt the water comes from the heat created by the kinetic energy of the skier, some of which is lost. The kinetic energy of the skier is reduced therefore the speed of the skier is lower. Even with the presence of a sufficient water layer, some dry friction is present. The graph below compares the total coefficient of friction with its components.

image from Lind and Sanders
The surface tension of water creates a force called capillary drag. In the picture below, the water is exerting a force on both the ski and the marble. In snow, the deeper the quasi-liquid layer on the ice crystals, and the thicker the layer of water melted by the skis, the stronger the drag force.

image from Lind and Sanders
With dryer, colder snow conditions, skis glide less easily, due to the temperature making it difficult for the lubricating water layer to form. It has been discovered though, that even in very cold conditions a layer of water is present. Oxygen atoms at the surface of ice and snow crystals vibrate faster than those in the interior. Because of this vibration an outer layer of molecule which acts like a liquid forms, making ice and snow slippery even at extremely cold temperatures.
When skiing, the constant rubbing of the skis against the snow creates a static electric charge, much like rubbing hair against a balloon. This static charge picks up any small particle on the trail such as pollen or dust. The presence of these contaminants on the bottom of the skis contributes to the over all friction, and can abrade the bottom of the skis and the wax, damaging them. The static charge also attracts the base of the ski to the snow, increasing the friction, contributing to the drag, this is termed Triboelectric drag