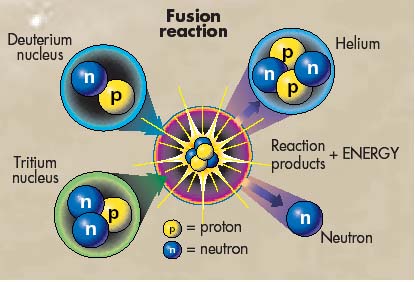
| Energy is created in a fusion reaction
through the loss of atomic mass from the beginning to the end of the reaction.
The mass of the two atoms is significantly more than the mass of the new
atom, which they fused together to form. This loss of mass is subsequently
converted into pure energy in the form of light and heat. The reason for
this amazing discovery is that mass is just a concentrated form of energy.
This understanding between the relationship of mass and energy was discovered
by Albert Einstein and illustrated in his famous equation E=mc^2, where
E is energy, m is mass, and c is the speed of light. Through this equation
the amount of energy held within a mass can be determined. In a plasma fusion
reaction between two hydrogen atoms the decrease in mass is about 4x10^-29
kg. This mass is then converted to energy, equaling 23.9 MeV. "To appreciate
the magnitude of this result note that if 1g of [hydrogen] is converted
to helium, the energy released… would be worth about $70,000"
(Physics for scientist and Engineers 1276). |
|
|
|
|
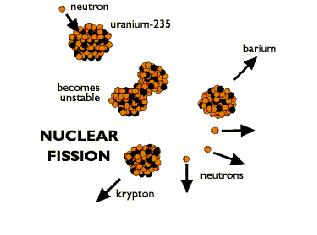
Fission
In a fission event an example of a reaction at an atomic level is an
(A)tomic-bomb. The A-bomb harnesses the power of an atom through an uncontrolled
reaction. This reaction is fission, which is the opposite of fusion. In
an A-bomb, atoms are being separated, not combined, which also creates
a massive release of energy but causes a large amount of nuclear waste
when harnessed as a source of power. Nuclear waste is created when any
type of matter comes in contact with an atom that has gone through a fission
reaction. The water that cools the system and the room that occupies the
reaction all become radioactive nuclear waste. The collision from the
neutron in the fission reaction causes the atom to become unstable, resulting
in a radioactive substance. From this instability the atoms become radioactive
and create instabilities within any other atom that they come in contact
with. Not only does fusion lack this step in its process, but through
fusion studies, scientists have been able to learn new and efficient ways
to clean up the process of fission.
|
|
|
|
Fusion
Waste
In both types of fusion reactions,
inertial and magnetic, the
wastes that are produced are basically the same. Before the fusion process
is started, deuterium (3^H) needs to be extracted from water molecules,
resulting in hydrogen and oxygen by-products. Both elements are absorbed
into the air with no negative effect. After deuterium is created the fusion
process can begin. When the deuterium and tritium atoms fuse together
they create a surge of energy. As a result of their fusing a helium atom
is created and a neutron is released (as illustrated in Fig. 1). Helium
is a completely harmless gas that can be released into the atmosphere,
but the neutron is currently a serious problem for plasma scientist.
After the neutron is released, due to the fusion of the 2^H and 3^H atoms,
it travels at a high velocity until it hits something. The neutron will
sometimes hit the lithium in the chamber, which creates more deuterium,
but most often it collides into the walls of the reactor. Since the neutron
is a neutrally charged particle it can travel through the magnetic fields
that surround the plasma. This is a serious problem for the chamber. From
the collisions of the neutrons the walls of the reactor break down and
become very brittle. One of the ways scientist are going about combating
this problem is by developing a new type of metal that can withstand a
neutron collision. This development is an example of the many spin-off
technologies that have been created to keep fusion technology moving into
the future.
|
|
|
|