|
What Is A
Superconductor?
A superconductor is a material
that can conduct electricity or transport electrons from one
atom to another with no resistance. This means no heat,
sound or any other form of energy would be released from the
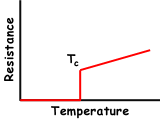
material when it has reached "critical temperature"
(Tc), or the temperature at which the material
becomes superconductive. Unfortunately, most materials must
be in an extremely low energy state (very cold) in order to
become superconductive. Research is underway to develop
compounds that become superconductive at higher
temperatures. Currently, an excessive amount of energy must
be used in the cooling process making superconductors
inefficient and uneconomical.
Superconductors come in two
different flavors: type I and type II. (1)
|
|
Type I Superconductors
A type I superconductor consists of
basic conductive elements that are used in everything from
electrical wiring to computer microchips. At present, type I
superconductors have Tcs between 0.000325 °K
and 7.8 °K at standard pressure. Some type I
superconductors require incredible amounts of pressure in
order to reach the superconductive state. One such material
is sulfur which, requires a pressure of 9.3 million
atmospheres (9.4 x 1011 N/m2) and a
temperature of 17 °K to reach superconductivity. Some
other examples of type I superconductors include Mercury -
4.15 °K, Lead - 7.2 °K, Aluminum - 1.175 °K
and Zinc - 0.85 °K. Roughly half of the elements in the
periodic table are known to be superconductive.
(1)
|
(http://www.superconductors.org/tc_graph.gif)
|

(http://www.superconductors.org/percht2.gif)
|
|
Type II Superconductors
A type II superconductor is
composed of metallic compounds such as copper or lead. They
reach a superconductive state at much higher temperatures
when compared to type I superconductors. The cause of this
dramatic increase in temperature is not fully understood.
The highest Tc reached at stardard pressure, to
date, is 135 °K or -138 °C by a compound
(HgBa2Ca2Cu3O8)
that falls into a group of superconductors known as cuprate
perovskites. This group of superconductors generally has a
ratio of 2 copper atoms to 3 oxygen atoms, and is considered
to be a ceramic. Type II superconductors can also be
penetrated by a magnetic field whereas a type I can not.
(2)
|
|
References
(1) http://www.superconductors.org/Type1.htm
(2) http://www.superconductors.org/Type2.htm
|
|
 Back to main page
Back to main page
|

