|
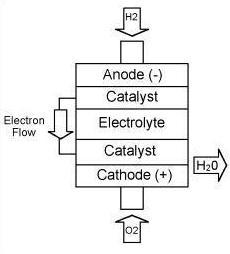
The principles behind the hydrogen fuel cell are fairly simple. Hydrogen
stored in the cell is combined with Oxygen in the air to produce water
and electricity. More specifically, the fuel cell is composed of
two electrodes which are coated with a catalyst such as platinum.
The platinum strips the electron from the hydrogen leaving an exposed proton.
The free electrons are conducted through the anode as a usable current.
The protons then proceed through the electrolyte medium to the cathode
where they react with the Oxygen in the air and electric current to produce
water and heat.
The current produced from this reaction can then be used to power and electric
motor |
