Spectrum Analysis
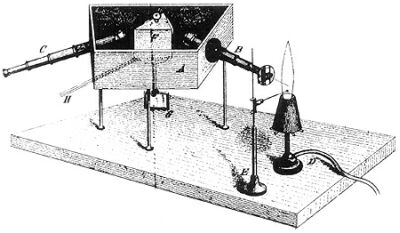
Kirchhoff was the first to explain the dark lines in the Sun's spectrum as caused by absorption of particular wavelengths as the light passes through a gas. He found that when light passes through a gas, the gas absorbs those wavelengths that it would emit if heated.
spectral lines - a discovery that began the spectroscopic method of chemical analysis.
Kirchhoff and Bunsen began by effectively inventing the spectroscope, a prism-based device that separated light in its primary chromatic components, i.e., its spectrum, with which they began studying the spectral "signature" of various chemical elements in gaseous form.

picture 7/10
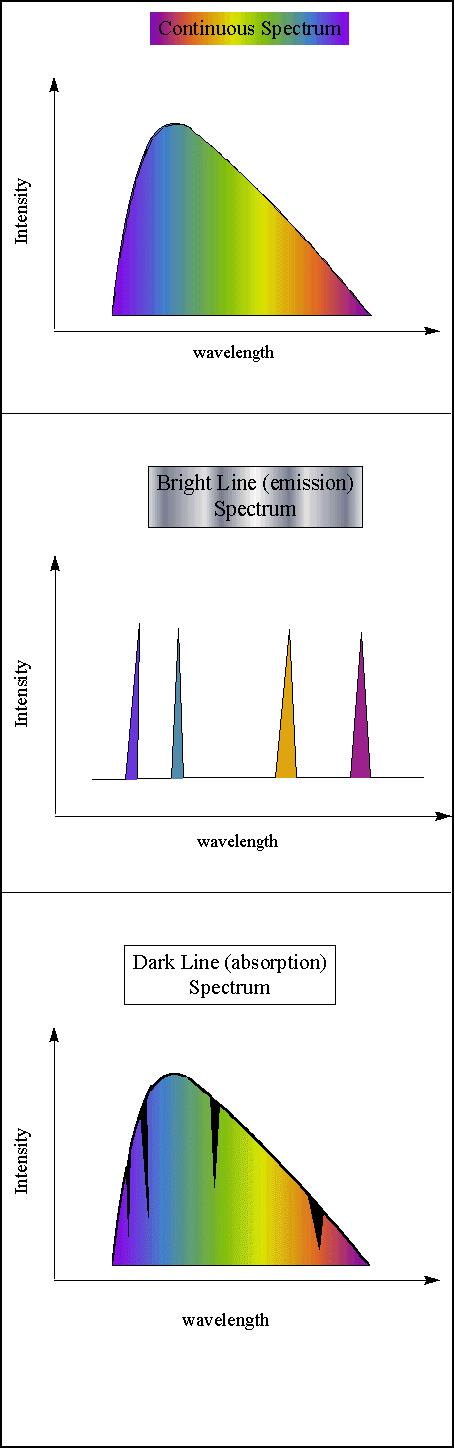 |
The spectrum of an object is the variation in the intensity of its radiation at different wavelengths. Objects with different temperatures and compositions emit different types of spectra. By observing an object's spectrum, then, astronomers can deduce its temperature, composition and physical conditions, among other things. Kirchhoff's Laws are:
|
Picture 9/10