
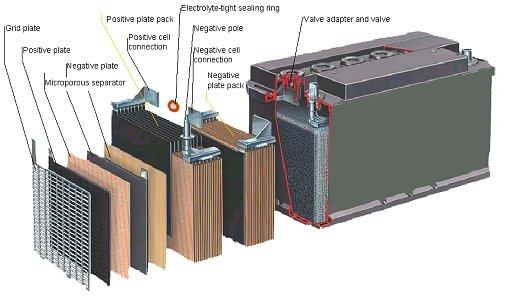
Automotive Battery
Both car batteries and deep cycle batteries are lead-acid batteries that use exactly the same chemistry for their operation. The difference is in the way that the batteries optimize their design:
A car battery typically has two ratings:
If you look at any battery, you'll notice that it has two terminals . One terminal is marked (+), or positive, while the other is marked (-), or negative. In an AA, C or D cell (normal flashlight batteries), the ends of the battery are the terminals. In a large car battery, there are two heavy lead posts that act as the terminals.
|


Electrons collect on the negative terminal of the battery. If you connect a wire between the negative and positive terminals, the electrons will flow from the negative to the positive terminal as fast as they can (and wear out the battery very quickly -- this also tends to be dangerous, especially with large batteries, so it is not something you want to be doing). Normally, you connect some type of load to the battery using the wire. The load might be something like a light bulb, a motor, or an electronic circuit like a radio.
Inside the battery itself, a chemical reaction produces the electrons. The speed of electron production by this chemical reaction (the battery's internal resistance ) controls how many electrons can flow between the terminals. Electrons flow from the battery into a wire, and must travel from the negative to the positive terminal for the chemical reaction to take place. That is why a battery can sit on a shelf for a year and still have plenty of power -- unless electrons are flowing from the negative to the positive terminal, the chemical reaction does not take place. Once you connect a wire, the reaction starts.
VOLTAGE
In any battery, the same sort of electrochemical reaction occurs so that electrons move from one pole to the other. The actual metals and electrolytes used control the voltage of the battery -- each different reaction has a characteristic voltage. For example, here's what happens in one cell of a car's lead-acid battery :
A lead-acid battery has a nice feature -- the reaction is completely reversible . If you apply current to the battery at the right voltage, lead and lead dioxide form again on the plates so you can reuse the battery over and over. In a zinc-carbon battery, there is no easy way to reverse the reaction because there is no easy way to get hydrogen gas back into the electrolyte.
BATTERY ARRANGEMENT
In almost any device that uses batteries, you do not use just one cell at a time. You normally group them together serially to form higher voltages, or in parallel to form higher currents. In a serial arrangement , the voltages add up. In a parallel arrangement , the currents add up. The following diagram shows these two arrangements:
|

The upper arrangement is called a parallel arrangement. If you assume that each cell produces 1.5 volts, then four batteries in parallel will also produce 1.5 volts, but the current supplied will be four times that of a single cell. The lower arrangement is called a serial arrangement. The four voltages add together to produce 6 volts.