Ice
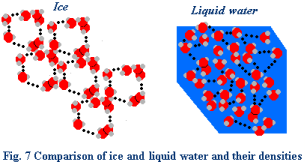
Water shows its strangest property when it transforms
from a liquid to a solid. In the
transformation to ice, it becomes less dense than liquid water. At temperatures above 4 Celsius, water
behaves like other liquids by expanding as it warms and contracting as it
cools. At temperatures below 4
Celsius, water begins to freeze and the molecules slow to a point where they do
not break their hydrogen bonds. A
crystalline structure forms at temperatures near 0 Celsius, with each water
molecule bonded to a maximum of four others. By holding molecules at a distance, it is the hydrogen bonds
that make ice less dense than liquid water (Fig. 7).